Ravshan K. Sultonov, Dilnoz B. Buriyeva, Xusnitdin B. Xujanov, Alisher R. Babamuratov
Department of Medicine, Termez University of Economics and Service, Uzbekistan
Correspondence to: Ravshan K. Sultonov, Department of Medicine, Termez University of Economics and Service, Uzbekistan.
| Email: |  |
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
In this article, the development dynamics and structure of tissue structures of the trachea and bronchial walls of infants under one year of age have been investigated. It has been proven that all the layers of the tracheal wall are not well developed at the age of one month of the babies, that the covering epithelium changes from multi-rowed to single-layered, the private plate changes from unformed connective tissue to forming tissue, and the larynx changes from a sparse chondroid and cellular structure to a dense chondromatous tissue with small cells.
Keywords:
Baby, Lung, Trachea, Bronchus, Postnatal ontogenetic, Histochemical, Histological, Hemotoxylin-eosin, Van-Gieson
Cite this paper: Ravshan K. Sultonov, Dilnoz B. Buriyeva, Xusnitdin B. Xujanov, Alisher R. Babamuratov, Estimation of the Dynamics of Development Indicators of the Trachea and Lung Bronches in Babies under One Year of Birth, American Journal of Medicine and Medical Sciences, Vol. 14 No. 3, 2024, pp. 548-555. doi: 10.5923/j.ajmms.20241403.03.
1. Introduction
According to the latest world data, the death of children is one of the main problems in the world's medical fields. Today's infant mortality rate is 15.6% per 1,000 live births, despite the fact that childbearing is very low, at 9.1%. [1]. Today, in developed countries, it is 5-12.5% before children. According to WHO data,more than 10% (15 million children) of some of the poor materials of our planet, this figure is 20% [2].In recent years, the level of air pollution has increased significantly, and there is a lot of evidence of this. It is possible to act against the environmental health effects of prenatal air pollution, especially lung bronchogenesis [3,4,5,18].An accurate representation of the anatomy of the human respiratory tract is essential for understanding and modeling the structure and functional relationships of healthy and patient lungs. For this purpose, seven generations of respiratory organs were examined by CT, MRI, and the diameter, length, and morphology of the airways were comprehensively studied on the basis of computer tomography [6,7].16 of the fetus; 17; At 18, 20, 28, 36 weeks and in 2 newborns, the trachea, bronchi, lungs, heart together with the pericardium were divided into age-related groups, and the area of the diaphragm surface was measured; length of pulmonary fissures; width; height; total lung volume was determined [8].The development of respiratory organs begins to progress in the third week of fetal life. The activity system of respiratory organs is inextricably linked with the activity of the cardiovascular system. The anatomical structure of the upper and lower respiratory tracts, their syntopy, is of great importance in predicting the clinical course of diseases and explaining the symptoms that appear [9].There are many theories about the structure of respiratory organs, their formation, perinatal and postnatal development [10,11,12,13,14].At the time of birth, the respiratory part of the respiratory tract is not yet fully formed: there are few alveoli, their wall is thicker than the alveolar wall of young people, and it contains very few elastic fibers. With the beginning of breathing, respiratory bronchioles begin to lengthen, new alveoli are formed in their walls. The size of the previously formed alveoli increases, barriers grow from their walls, which divide the cavity into parts - new alveoli are formed. The most active alveologenesis takes place in the first 18 months of a child's life, the formation of new alveoli continues until the age of 5. The correct formation of alveoli is caused by the active development of microcirculatory flow and formed elastic fibers in the presence of a complete surfactant system. The full development of the elastic structure in the lungs is completed only by the age of 18 [15,16].As a result of long-term ingestion of small amounts of toxic substances or repeated acute poisoning, as well as material or functional accumulation of poison, some toxic substances that selectively affect the body during postnatal ontogenesis, causing morphological and functional changes in individual organs and systems (respiratory, reproductive, hematopoietic, etc.) comes [17].As can be seen from the information in the above literature, the dynamics of development indicators of the trachea and lung bronchi in babies under one year old have not been evaluated.
2. Purpose of the Research
It consists in the study of indicators of postnatal ontogenetic development of trachea and pulmonary bronchi in babies under one year of age.
3. Materials and Methods
The examination was conducted on the corpses of infants under one year of age who arrived in 2020-2022 at the Center for Pathological Anatomy of the Republic. In dead children, the lungs were studied in cadavers of children who died mainly due to heart defects and other causes without diseases of the bronchial passages. The causes of death and the main disease were determined in the results of pathological anatomy. Examination materials were obtained in the following parts of the lungs: Trachea, right and left internal bronchi were studied by opening the bronchi from the lobes to the terminal bronchi. Instrumental (with the help of barbell), general histological, histochemical, morphometric and statistical research methods were used in our research. The obtained materials were fixed in formalin and then 3-5 µm sections were prepared. They were stained with hemotoxylin- eosin, Schick, Van-Gieson reaction methods. | Figure 1. Taking the size of the bronchi of a 3-month-old baby using an electronic barometer |
4. Results and Discussion
For the study, the corpses of infants under 1 year of age who did not have any diseases in the respiratory system, died mainly from heart attacks and various injuries, were taken, and the pathologoanatomical materials taken from the parts of the trachea (throat), intrapulmonary bronchi from all the infant corpses were subjected to instrumental, histochemical, and morphological examinations. For the examination, the diameters of the right and left lungs of all infants were measured: bronchioles, bronchioles, and terminal bronchioles.A period of one month. Before the age of 1 month, the bronchi of the lung lobes of newborn babies are funnel-shaped, the length is 3.7±0.8 cm, and the width of the cavity is on average 0.7±0.1 cm. The wall is relatively soft, that is, the ankles are less developed, thin and soft. It was found that the entire structure of Togai halkalkas was broken and consisted of several pieces.It was observed that the Togay's skin is relatively thin and flat, and has an immature structure, that is, the cells are small, numerous and densely located, and the cytoplasm of some of them appears to be vacuolated. It was found that the main substance of the togai is dense and darkly colored, and the inner and outer surface of the togai is covered with tissue bundles connecting the togai rings.It is surrounded by a thin and swollen serous membrane on the outside. The mucous membrane is folded, but the folds are flatter than the folds of the trachea and have a smaller structure (see Fig. 2). Few goblet cells were found among the covering epithelium. It was observed that the basal membrane and private plate consist of connective tissue with many dense fibers and relatively few cells. In the submucosal layer, adjacent to the private plate, a tuft of smooth muscle cells is found. The submucosa was found to consist of thin, light-stained, unformed connective tissue with very little swelling of fibers and cells. There are serous- mucous glands in the submucosa, their number and immature structure compared to the trachea was observed.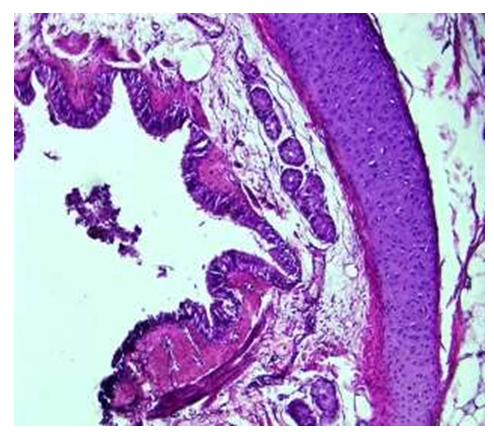 | Figure 2. Bronchi of lung lobes, period of 1 month. The folds of the mucous membrane are relatively small, the submucous layer is thin, there are few tissue structures, and the mucous membrane is multicellular. Paint: G-E. Zoom: 10x40 |
Microscopic examination of intralobular bronchioles for one month showed that they are located between segments of lung tissue, surrounded by blood vessels and nerve fibers, separated from lung tissue by a thin and thin connective tissue membrane. It was observed that the mucous membrane of the bronchiole consists of large and small folds, and the large folds are symmetrically located on both sides of the bronchial wall (see Fig. 3).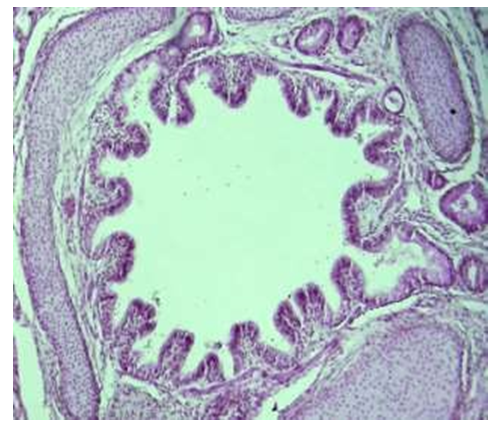 | Figure 3. Bronchi of lung lobes, period of 1 month. It is observed that the epithelium and glands covering the bronchial wall contain a small amount of positive substance. Paint: staining with Schiff's reagent-reaction. Zoom: 10x10 |
When examining the wall of a segmented bronchiole under a large lens of a microscope, the following was revealed. It was found that the covering epithelium in the upper part of the folds of the mucous membrane has a thick and multi-rowed structure. In the pits of the folds, it was observed that the covering epithelium has a relatively thin structure. Covering epithelial nuclei were found to be randomly arranged and stained with dark hyperchromasia. The private plate of the mucous membrane has a relatively fine structure, and contains many thin-walled blood vessels. In the submucosa layer, compared to the wall of the bronchus shown earlier, it was observed that the tuft of smooth muscle cells was thick and branched, connected with the private plate and the connective tissue on the side of the larynx.Microscopic examination of tissue structures of the terminal bronchiole wall in one month showed that it was different from the wall of the bronchus and bronchioles shown earlier, that is, it was relatively thin and consisted only of mucous membrane and submucous muscle layer. In the mucous membrane, it was found that there are only folds arising from the long-shortness of the covering epithelium. From the covering epithelial cells, it was found that the folds are cylindrical in the protruding areas, and prismatic in the pits (see Fig. 4). Histochemical examination of the fibrous structures of the connective tissue in the wall of the terminal bronchiole revealed that it consisted of densely located picrofuchsin-positive fibers attached to the alveolar tissue around the outer layer of the covering epithelium. These connective tissue fibrous structures were found to be relatively thickened toward the vascular side around the bronchioles, and the vascular wall was fused with connective tissue fibers (see Figure 5).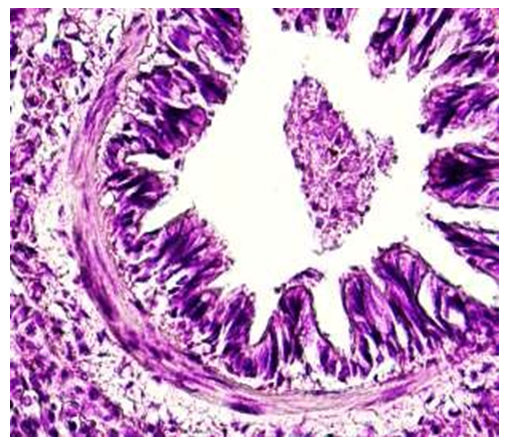 | Figure 4. Terminal bronchiole. A period of one month. The wall of bronchioles consists of covering epithelium, thin private plate and smooth muscle layers. Paint: G-E. Zoom: 10x40 |
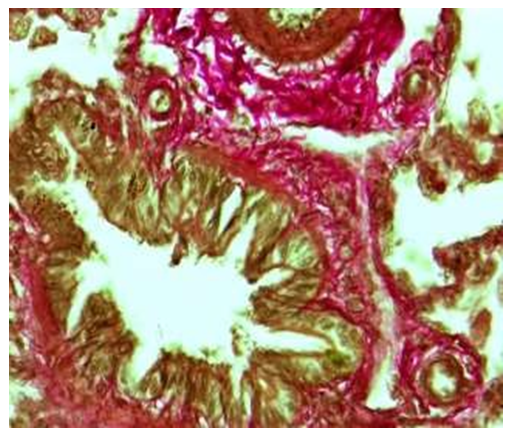 | Figure 5. Terminal bronchiole. A period of one month. Picrofuchsin-positive staining of fibrous structures in the wall of the bronchioles was intermingled with the fibers of the vascular wall. Paint: Van-Gieson method.. Zoom: 10x40 |
3-month period. It was found that in this period, the bronchi of the lung lobes of the babies became tube-like, their length was slightly increased, that is, on average 4.2±0.9 cm, the width of the cavity was on average 0.8±0.02 cm. The thinness of the wall is preserved, it was found that the larynx is still underdeveloped, the wall is thin and soft. It was found that Togay halkalkas consist of several pieces.Microscopic examination of intra-lobular bronchioles in this period of infants showed that they were observed to be located between segments of lung tissue. It was observed that the folds in the mucous membrane of the bronchiole were almost gone, and in some places, small and flattened small pieces were preserved. It was found that the covering epithelium has become larger and cylindrical in shape compared to previous periods, and its cytoplasm has expanded, and there is a mucous substance on its surface (see Fig. 6).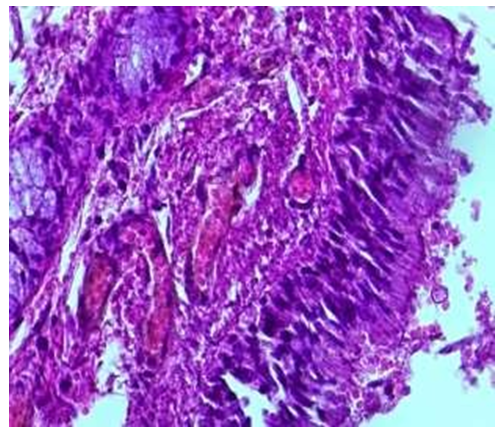 | Figure 6. Intralobular bronchiole, period of 2.5-3 months. The covering epithelium is cylindrical, the private plate is rich in fibers, blood vessels are expanded. Paint: G-E. Zoom: 10x40 |
In the period of 3 months, the microscopic examination of the wall structures of the terminal bronchioles showed that, compared to the previous periods, the covering epithelium was thinner, smaller in size, and took on a prismatic shape. It was observed that the nuclei of these cells were relatively dark and irregularly arranged. It was observed that under the covering epithelium, there is an unformed connective tissue with relatively increased fibers and irregularly arranged cells, and that the connective tissue is connected to the wall of blood vessels around the bronchioles. When the wall of the terminal bronchiole is studied under a microscope, it is found that the covering epithelium is relatively small, prismatic, and has a multi-row structure. It is determined that the private plate is located directly adjacent to the smooth muscle layer. It is determined that several rows of smooth muscle cells are connected to form a relatively large tuft, which surrounds the wall of the bronchiole in a circle. From the outside, it was found that the blood vessels adjacent to the lung tissue were surrounded by a lot of connective tissue (see Fig. 7).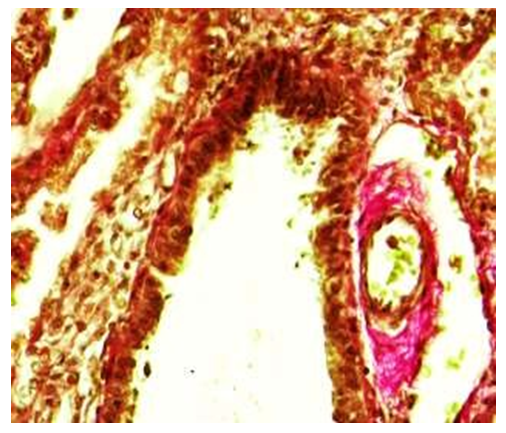 | Figure 7. Terminal bronchiole. 3 month period. There are no fibers positively stained with picrofuchsin in the wall of bronchioles, only in the wall of surrounding blood vessels. Staining: Van-Gieson method. Zoom: 10x40 |
6 month period. By this period of the examination, it was found that the inner bronchi of the lung lobes kept their tubular shape, their length was slightly elongated, that is, on average 4.4±0.9 cm, and the width of the cavity was on average 0.26±0.2 cm. The thickness of the bronchial wall is preserved, the bronchial tubes are relatively improved, the wall is thin and soft. It was found that Togay halkalkas consist of several pieces.By the 6-month period, it was found that the togai people were slightly flattened, thickened, relatively immature in terms of composition, that is, the cells were small, numerous and densely located, only some of them had a vacuolated cytoplasm. It was found that the intermediate chondroid material of the togai is dense and darkly colored, and the tissue bundles connecting the togai rings from the inner and outer surface are relatively increased. It is surrounded by a thin and swollen serous membrane on the outside. It was found that the folds of the mucous membrane have disappeared, smoothed, the surface is covered with a single- layered multi-row cylindrical epithelium, the height of the epithelium has decreased in some areas, depressions have appeared. Among the covering epithelium, goblet cells were slightly increased compared to the previous period.Microscopic examination of the bronchioles of 6-month-old infants showed that they are located between the segments of the lung tissue. It was observed that the larynx differed from the bronchi and mainly consisted of separate large and small pieces. It was found that the composition of the cartilage consists of numerous and small chondrocytes, and the interstitial material consists of relatively dense and darkly colored chondroid material. It was found that the blood clots on the private plate were relatively expanded, filled with blood, and as a result, the wall was thickened compared to the previous period. It was found that the muscle bundles formed two- and three-layered bundles, and in some places they were multi-layered. It was observed that the folds in the mucous membrane of the bronchiole were almost gone, and in some places, small and flattened small pieces were preserved. It was found that the covering epithelium has become larger and cylindrical in shape compared to previous periods, and its cytoplasm has expanded, and there is a mucous substance on its surface (see Fig. 8).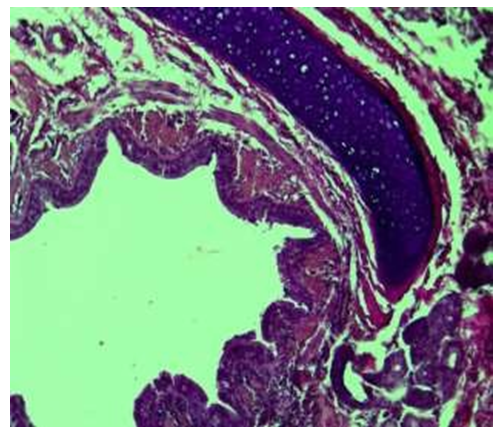 | Figure 8. Intralobular bronchiole, 6 months period. Covering epithelium is cylindrical, rich in special plate fibers. Paint: G-E. Floor: 10x40 |
During the 6-month period of the study, the microscopic examination of the wall structures of the terminal bronchioles showed that, compared to the previous periods, the covering epithelium was thinner, smaller in size, and took on a prismatic shape. It is observed that the nuclei of covering epithelial cells are relatively darkly stained and randomly arranged. In the private plate, it was observed that there is a relatively increased number of fibers, the presence of unformed connective tissue with irregularly arranged cells, and that the connective tissue is connected to the wall of blood vessels around the bronchiole.Histochemical examination of the fibrous structures of the connective tissue in the wall of the terminal bronchioles of the 6-month-old infants showed that they were relatively increased in the surrounding alveolar tissue after the outer layer of the covering epithelium and consisted of densely located fibers positively stained with picrofuchsin. These connective tissue fibrous structures were found to be relatively thickened towards the vascular side around the bronchioles, and the vascular wall was fused with connective tissue fibers (see Figure 9).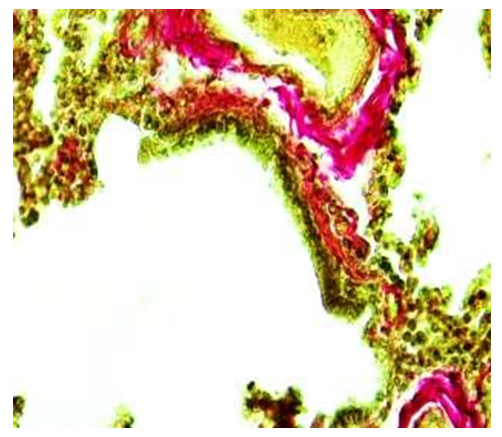 | Figure 9. Terminal bronchiole. 6 month period. There are few fibers positively stained with picrofuchsin in the wall of the bronchioles, and there are many around the blood vessels. Staining: Van-Gieson method. Zoom: 10x40 |
When infants were 12 months old, the following data were obtained when the walls of the lung lobes were examined microscopically. It was found that the lining epithelium of the bronchial mucosa is covered with a single-layer cylindrical epithelium, and there are no folds on the surface, the lining epithelium is everywhere, and there are deep pits of the epithelium everywhere. The covering epithelium in these areas is relatively low and its cytoplasm is found to be vacuolated. It was found that the connective tissue under the basement membrane merged with the private plate, forming a relatively thick layer (see Fig. 10).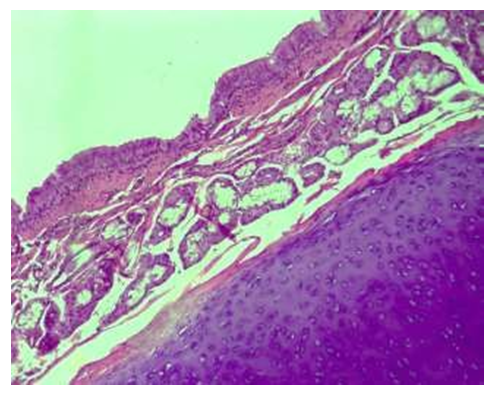 | Figure 10. 12-month period. Lung lobes are the main bronchus, the larynx is dense, there are relatively many glands, and the private plate is thin. Paint: G-E. Zoom: 10x40 |
Microscopic examination of the bronchioles of the lung lobe of 12-month- old infants showed that these bronchioles were observed to be located between the segments of the lung tissue. It was observed that the walls of the bronchioles are different from those of the bronchioles and mainly consist of large and small pieces located separately. It was found that the composition of the tissue of Togay consists of numerous and small chondrocytes, and the interstitial substance consists of relatively dense and darkly colored chondroid substance. It was found that the blood clots on the private plate are relatively numerous, and most of them are enlarged, filled with blood, and as a result, the wall is thickened compared to the previous period. It was observed that the muscle bundles formed two- and three- layered bundles, and in some places they were multi-layered.During the 12-month period of the study, the microscopic examination of the wall structures of the terminal bronchioles in the lung tissue showed that, compared to the previous periods, the covering epithelium was thinner, smaller in size, and took on a prismatic shape. It is observed that the nuclei of the epithelial cells covering the mucous membrane are relatively darkly stained and randomly arranged. In the private plate with connective tissue, there are relatively few fibers, connective tissue cells are randomly located, there is an unformed connective tissue everywhere, and it is observed that the connective tissue is connected to the blood vessel wall around the bronchiole. When the lung tissue wall of the terminal bronchiole is studied under a microscope, it is found that the covering epithelium is relatively small, prismatic, and has a multi-row structure. It is determined that the special plate with connective tissue is located directly adjacent to the smooth muscle layer. It is determined that several rows of smooth muscle cells are connected to form a relatively large tuft, which surrounds the wall of the bronchiole in a circle. From the outside, it is observed that the blood vessels adjacent to the lung tissue are surrounded by a lot of connective tissue.At the age of 12 months, the histochemical examination of the fibrous structures of the connective tissue in the wall of the terminal bronchiole showed that it was relatively increased and consisted of fibers positively stained with picrofuchsin, attached to the alveolar tissue around the outer layer of the covering epithelium. It is found that these connective tissue fibrous structures are relatively thickened towards the side of the blood vessels around the bronchioles, and the vascular wall is fused with connective tissue fibers (see Fig. 11).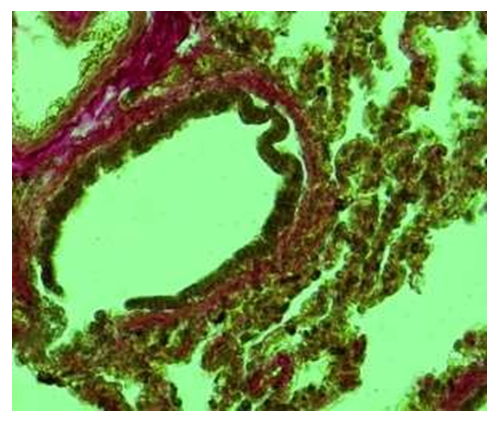 | Figure 11. Terminal bronchiole. 12-month period. There are few fibers stained positively with picrofuchsin in the bronchial wall of the bronchial lobe, and there are many around the blood vessels. Staining: Van-Gizon method. Zoom: 10x40 |
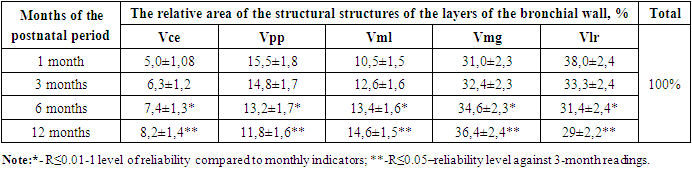
It is known that the connective tissue private plate of the mucous membrane of the bronchial wall has an unformed structure in the 1st month of infancy, its interstitial substance nardon and swelling processes prevail, and it was observed that its thickness was 15.5±1.8% of the thickness of all layers of the bronchial wall. In the following months of the early postnatal period, due to the relatively improvement of cells and fibrous structures in the composition of the unformed connective tissue in the private plate of the mucous membrane, and the reduction of the swelling process in the intermediate substance, its relative thickness at 3 months is 14.8±1.7%, at 6 months it is -13.2± 1.7%, at 12 months - 11.8±1.6% reduction was found. The smooth muscle layer of the bronchial wall, on the contrary, in the early postnatal period, starting from the 1st month, it was observed that in the following months, due to the increase in both the number and the size of the muscle cells, it regularly thickened. It was found that it thickened by % and reached 14.6±1.5%.In the early postnatal period of babies, one of the structures in the wall of the respiratory tract that performs another important function is the mucous gland structures that synthesize mucus. According to the results of our research, both morphologically and morphometrically, these glands grow and multiply in the dynamics of the early postnatal period, and the area occupied by them increases. During 1 month, it was found that the wall of the bronchus occupied one third of the area of all its layers, i.e. 31.0±2.3%. It was found that in the next period of the examination, that is, 32.4±2.3% in the 3-month period, 34.6±2.3% in the 6-month period, and 36.4±2.4% in the 12-month period. So, in general, by the end of the early postnatal period, it was observed that the area expanded by 5.5%.It was found that the relative occupied area of the bronchial tubes in the bronchial wall was 38.0±2.4% of the area of all layers in 1 month of the examination. Due to the relatively large area of the condyle, the histological examination revealed that the chondrocyte cells in its composition were young and not well developed, and as a result, it was relatively large, the chondroid material between them was relatively pale, and the swelling process prevailed. In the following months of the early postnatal period, both the cells and the intermediate chondroid substance in the umbilical cord were improved and differentiated, so they became denser and their occupied area decreased, and by the 12th month period, it was found that they occupied 29±2.2% of the space.Table 1. Morphometric indicators of structural units of bronchial wall layers in the dynamics of the early postnatal period, %
 |
| |
|
5. Conclusions
It was confirmed that the wall of the main bronchus of the lung lobes changes from a funnel-shaped shape to a cylindrical shape, the covering epithelium from a multi-rowed, folded state to a single-layer smooth structure, the private plate from a multicellular unformed state to a fibrous structure-formed connective tissue, and the larynx from a flat and swollen appearance to a round and dense appearance.The layers of the wall of the bronchial bronchioles are formed from incompletely formed to perfected at the age of one month, the covering epithelium is wrinkled, the goblet cells are smooth from few, goblet cells are abundant, the private plate is swollen, and the fibers and muscle bundles are arranged in an orderly manner from the unformed connective tissue. It was found that the elongated two-lobed to four-lobed dense state has changed.It has been proven that the walls of terminal bronchioles in babies are semi- circular in shape, one side is lined with cylindrical epithelium, and the other side is adjacent to the alveoli. By the age of one year, the covering epithelium is transformed into a prismatic type, a whole bundle consisting of connective tissue and muscle fibers with a special plate.In the 1st month of the early postnatal period, the area occupied by the covering epithelium was 1/20 of the area of all layers of the bronchial wall, and by the 12th month, it was thickened to 11/1, the muscular layer was 9.5/1 to 6.5/1, the mucous glands were 1/3 to 2 It was observed that the private plate was thickened from .7 to 1 part, on the contrary, the private plate was reduced from 6.4/1 to 8.5/1 parts, and the condyles were narrowed from 2.6/1 to 3.4/1 parts.
References
| [1] | Sultanov. R.K, Sodikova. Z.Sh, Arsenova. M.A, Boboyorov. S.U. Morphological and Morphometric Indications of Trachea and Bronchial Walls in One-Month-Old Babies. // American Journal of Medicine and Medical Sciences 2022, 12(8): P-811-814. (14.00.00; №2). https://scholar.google.com/citations?view_op= view_citation&hl=ru&user=Ok1KQNIAAAAJ&citft=1&citft=2&citft=3&email_for_op=ravshansultonov606%40 gmail.com&citation_for_view=Ok1KQNIAAAAJ:UebtZRa9Y70C. |
| [2] | Baranov, A.A. State of health of children in the Russian Federation / A.A. Baranov // Pediatrics. - 2012. - T. 91, No. 3. - pp. 9-14. |
| [3] | Insa Korten, Kathryn Ramsey, Philipp Latzin. Air pollution during pregnancy and lung development in the child. Paediatric Respiratory Reviews 21 (2017) 38–46. |
| [4] | Cindy T. McEvoy, MD, MCR, Eliot R. Spindel, MD, PhD. “Pulmonary Effects of Maternal Smoking on the Fetus and Child: Effects on Lung Development, Respiratory Morbidities, and Life Long Lung Health”. Paediatr Respir Rev. 2017 January 21. |
| [5] | Sultanov R.K., Sodikova Z.Sh., Kamalova G.B "Morphometric parameters of the larynx wall in one-month-old babies" // TMA Vestnik, 2022. pp. 319-320 https://scholar.google.com/citations?view_op=view_citation &hl=ru&user=Ok1KQNIAAAAJ&citft=1&citft=2&citft=3&email_for_op=ravshansultonov606%40gmail.com &citation_for_view=Ok1KQNIAAAAJ:Se3iqnhoufwC. |
| [6] | Van de Moortele T, Wendt CH, Coletti F. Morphological and functional properties of the conducting human airways investigated by in vivo computed tomography and in vitro MRI.// J Appl Physiol (1985). 2018 Feb 1; 124(2): 400-413. |
| [7] | Nefedov S.V., Chernyaeva T.M., Torchilo S.M., Sattieva Ya.R. Ultrasound diagnosis of the lungs in premature newborns // Neonatology: news, opinions, training. - 2020. - V. 8. No. 1 (27). - S. 61-66. |
| [8] | Kuznetsova A.V. On the issue of dyschronism of lung development // Children's Medicine of the North-West. 2018. V. 7. No. 1. S. 182-183. |
| [9] | Basiy R.V., Vasiliev V.A., Zdikhovsky I.A., Dovgyallo Yu.V., Beshulya O.A., Selivanova E.S. Anatomy of the lungs // Bulletin of hygiene and epidemiology. - 2018. - V. 22. No. 4. - pp. 87-90. |
| [10] | Blinova S. A., Yuldasheva N. B., Khotamova G. B. Vegetative innervation of the lungs in postnatal ontogenesis // Problems of science and education. 2021. No. 19 (144). |
| [11] | Lebedko O.A., Ryzhavsky B.Ya., Guseva O.E., Lazovskaya O.V., Kuznetsova M.S. Influence of echinochrome a on lipopolis-charide-induced lung damage at an early stage of postnatal ontogenesis (in experiment) // Rosvestn Perinatol and Pediatrics. 2017. No. 4. –pp.206. |
| [12] | Mikhailova D.D., Rychkova A.A. Structural and electron-microscopic characteristics of the human lung in the embryonic and early fetal periods of prenatal ontogenesis // Actual problems of theoretical, experimental, clinical medicine and pharmacy. Materials of the 51st All-Russian scientific conference of students and young scientists. 2017. - Publisher: RIC "Iveks". - pp. 188-189. |
| [13] | Moldavskaya A. A, Gaziev M.A. Morphogenesis and topographic and anatomical features of the lungs in human prenatal ontogenesis // Astrakhan Medical Journal. -2012. -№4. -pp. 188-190. |
| [14] | Mirsharopov. U.M. “Morphological transformations of extraorganic and intraorganic human pulmonary veins in postnatal ontogenesis.” Abstract of the dissertation for the degree of doctor of medical sciences. |
| [15] | Tronina D.A., Tsoi E.G. Early diagnostic criteria for bronchopulmonary dysplasia in premature newborns // In the collection: Kuzbass: education, science, innovations. Materials of the Innovation Convention. Department of Youth Policy and Sports of the Kemerovo Region, Kuzbass Technopark, Council of Young Scientists of Kuzbass. 20355-357. |
| [16] | Studenikina, T. M. Embryogenesis and early postnatal development of human tissues and organs: textbook. -method. allowance. - Minsk: BSMU, 2020. – pp. 52. |
| [17] | Mirsharapov U.M., Sagatov T.A., Khuzhanazarova S.Zh. Features of intraorgan veins M.T. in women of mature, elderly and senile age. // Morphology. -2008. - No. 2. pp - 88. |
| [18] | Sultonov, R. K., Sodiqova, Z. S., & o’g’li, B. S. U. (2021). Dynamics of Fat Cells of the Bronchial Tree Mucosa in Postnatal Ontogenesis. Central Asian Journal of Medical and Natural Science, 2(4), 182-184. https://doi.org/10.47494/cajmns.v2i4.271. |







 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML





