Sadulloeva I. K.
Bukhara State Medical Institute, Bukhara, Uzbekistan
Correspondence to: Sadulloeva I. K., Bukhara State Medical Institute, Bukhara, Uzbekistan.
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
Congenital heart defects (CHDs) are structural changes in the heart's structure that form during the development of the fetus in the womb. These defects may affect the structure of the heart, the valves, the walls of the heart, or the large vessels leading to and from the heart. Children with congenital heart disease may have an altered immune status, especially if the defect is associated with severe impairment of blood circulation and heart function. Determination of characteristics of the content of growth factors (bFGF, VEGF-A and TGF-β) in blood serum in children with white and blue types of congenital heart disease. Included 52 children with an established diagnosis of white (28 patients) and blue (24 patients) congenital heart disease. The control group consisted of 28 practically healthy children of the same age. The concentration of fibroblast growth factor (bFGF), vascular endothelial growth factor-A (VEGF-A) and transforming growth factor-beta (TGF-β) in blood serum was determined by enzyme-linked immunosorbent assay using test systems of JSC Vector-Best (Novosibirsk, Russia). Hypersecretion of bFGF, VEGF-A and TGF-β was established in patients of both groups with congenital heart disease. Hypoxia, ischemia, compensatory angiogenesis, and cardiac tissue depletion involve the activation of bFGF, VEGF-A, and TGF-β, which play an important role in tissue regeneration and remodeling.
Keywords:
Congenital heart defects, Children, Growth factors, Cyanosis, Cytokines, Serum, Imbalance
Cite this paper: Sadulloeva I. K., Features of Serum Content of Growth Factors in Congenital Heart Defects of Different Types in Children, American Journal of Medicine and Medical Sciences, Vol. 14 No. 2, 2024, pp. 499-504. doi: 10.5923/j.ajmms.20241402.68.
1. Introduction
Congenital heart defects (CHDs) are structural changes in the heart's structure that form during the development of the fetus in the womb. These defects may affect the structure of the heart, the valves, the walls of the heart, or the large vessels leading to and from the heart [1].The most common defects are the following: ventricular septal defect – VSD (28.3%); atrial septal defect – ASD (10.3%); pulmonary stenosis (9.8%); tetralogy of Fallot – TF (9.7%); aortic stenosis (7.1%); coarctation of the aorta (5.1%); transposition of the great vessels (4.9%); Tricuspid valve hypoplasia syndrome, patent ductus arteriosus (PDA), and complete anomalous venous return also occur [8]. More than 90 variants of congenital heart disease and many of their combinations are observed.At the Institute of Cardiovascular Surgery named after. A.N. Bakulev developed a classification based on the distribution of congenital heart disease, taking into account the anatomical features of the defect and hemodynamic disorders. For practicing cardiologists, it is more convenient to use a more simplified division of congenital heart disease into 3 groups:• Pale type congenital heart disease with arteriovenous shunt: VSD, ASD, PDA; open atrioventricular canal (AVC).• Blue type congenital heart disease with venoarterial shunt: TMS, TF, Fallot's triad, tricuspid valve atresia, etc.• CHD without shunt, but with an obstruction to the blood flow from the ventricles (pulmonary artery and aortic stenosis). This division covers the 9 most common congenital heart defects [2,6].Children with congenital heart disease may have an altered immune status, especially if the defect is associated with severe impairment of blood circulation and heart function [3].Based on the above, the purpose of this study is to determine the characteristics of the content of growth factors in blood serumin children with white and blue types of congenital heart disease.
2. Material and Methods
The present study included52 childrenwith an established diagnosis of white (28 patients) and blue (24 patients) congenital heart disease.The control group consisted of 28 practically healthy boys and girls of the same age. The study involved children aged 1 to 12 years.Immunological studies of the examined women and men were carried out in the immunoregulation laboratory of the Institute of Human Immunology and Genomics of the Academy of Sciences of the Republic of Uzbekistan.The concentration of basic fibroblast growth factor (bFGF), vascular endothelial growth factor-A (VEGF-A) and transforming growth factor-beta (TGF-β) in peripheral blood serum was determined by enzyme-linked immunosorbent assay using test systems of JSC "VECTOR- BEST" (Russia, Novosibirsk). Quantitative assessment of the results was carried out by constructing a calibration curve that reflects the dependence of optical density on concentration for the standard antigen and allows comparison of the test samples with it.Statistical processing of the obtained data was carried out using the computer program “Statistica 6.0”.Data were statistically processed using conventional approaches, and results are presented as sample mean (M) and standard error of the mean (m); median (Me), characterizing the central tendency, and upper and lower quartiles, characterizing the spread of indicator values among 50% of respondents (Q1-Q3), where Q1 is the 25% percentile, Me is the 50% percentile, Q3 is the 75% percentile. The reliability of differences in mean values (P) of the compared indicators was assessed using the Student's t test (t).
3. Results and Discussions
As you know, the normal functioning of tissues depends on the regular supply of oxygen by blood vessels. The process of neoangiogenesis is necessary for long-term adaptation of tissues under conditions of damage [12].When tissue damage and inflammation occur, fibroblasts are activated by macrophages, secrete fibroblast growth factors (bFGF), and then actively migrate to the site of damage, binding to fibrillar structures through fibronectin, while simultaneously synthesizing extracellular matrix substances [14].Fibroblasts secrete proangiogenic factors - vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), transforming growth factor beta (TGFβ) [13].Growth factors, commonly thought of as a series of cytokines, are proteins that stimulate cell growth, differentiation, survival, inflammation, and tissue repair. Growth factors are important for the regulation of various cellular processes, which can be secreted by neighboring cells, distant tissues and glands, or even the tumor cells themselves. Normal cells require several growth factors to maintain proliferation and viability. Growth factors can exert stimulation through endocrine, paracrine or autocrine mechanisms [10].Growth factors are involved not only in the processes of remodeling angiogenesis, the immune response, but alsocan serve as biomarkers, which, under certain pathological conditions, partially enter the blood, which has diagnostic value [10,12].The results obtained are presented in Table 1. below.Table 1. Serum growth factor levelsamong those examined sick children with congenital heart disease
 |
| |
|
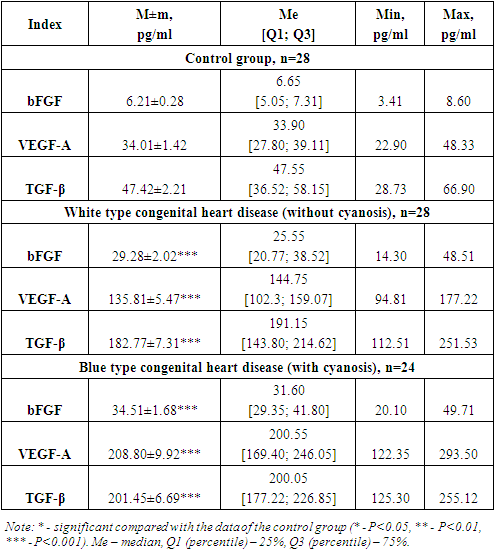
Fibroblast growth factor (FGF-fibroblast growth factor) are a family of cellular signaling proteins produced by various cell types [9]. One of the main functions is to stimulate the proliferation and migration of cells, including fibroblasts, endothelial cells and cardiac myocytes.As shown in Fig. 1, the analysis of serum bFGF concentration established significantly significant indicators. Thus, the level of this growth factor was increased by 4.7 times, which averaged 29.28 ± 2.02 pg/ml, with an individual range from 14.3 to 48.5 pg/ml, whereas in the group of healthy children this the mean value was 6.21±0.28 pg/ml (P<0.001).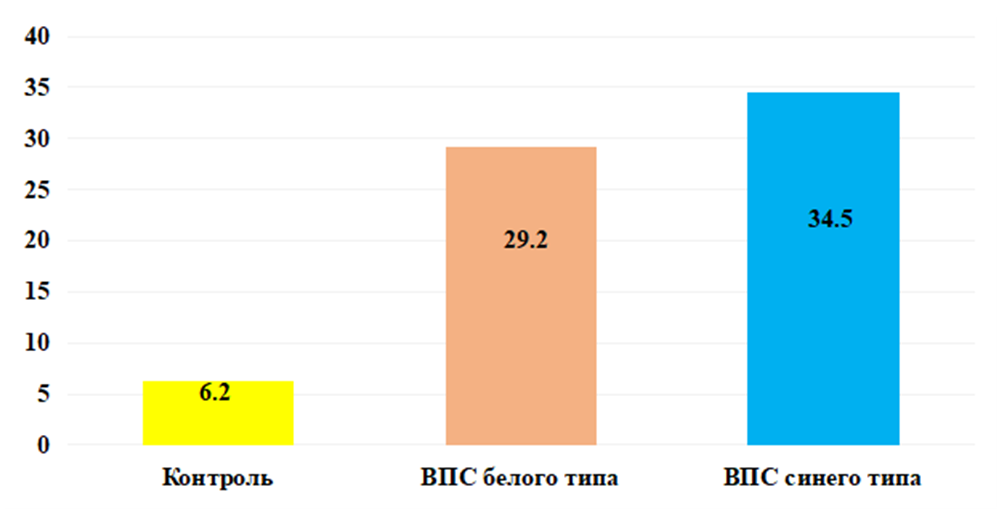 | Figure 1. Serum level of bFGF in examined children in comparison. Note: * - significant compared with the data of the control group (* - P<0.05, ** - P<0.01, *** - P<0.001) |
Elevated serum levels of bFGF are likely due to tissue stress and injury. Also, increased synthesis of bFGF is likely involved in this process to compensate for the lack of blood supply in certain areas of the heart, which activates the process of angiogenesis and releases bFGF to stimulate the growth of new blood vessels, thereby stimulating and remodeling cardiac tissue, which is likely already experiencing wall thickening and hypertrophy myocardium.As shown in Fig. 1, analysis of the serum concentration of bFGF established significantly significant indicators in the group of children with blue type CPPS. Thus, the level of this growth factor was increased by 5.6 times, which averaged 34.51 ± 1.68 pg/ml, with an individual range from 20.1 to 49.7 pg/ml, whereas in the group of healthy children this the mean value was 6.21±0.28 pg/ml (P<0.001).It is likely that elevated values indicate chronic hypoxia, which could trigger increased expression. Just as under conditions of chronic hypoxia and tissue damage, fibrosis can develop, which can affect the structure and function of the heart, promote angiogenesis, activate inflammatory processes and tissue remodeling, which are part of the immune response and can affect the regulation of blood vessels and pressure in the heart and blood vessels, which may also be important for congenital heart disease.Of the many proangiogenic factors involved in physiological and pathological angiogenesis, VEGF is the most important mediator of vascular endothelial growth. VEGF is a family of structurally related proteins that, together with receptors (VEGFR), play a leading role in the development and regulation of the activity of blood and lymphatic vessels. The VEGF family of molecules includes several factors: VEGF-A, -B, -C, -D, -E and placental growth factor PlGF [11]. VEGF-A, -B and PlGF are the main regulators of blood vessel growth, VEGF-C and -D are necessary for the formation of lymphatic vessels [7,11].A key regulator of angiogenesis is VEGF-A. Being a glycosylated mitogen, it specifically affects endothelial cells and has pleiotropic effects, including mediating an increase in the permeability of the vascular wall, inducing vasculogenesis, angiogenesis and growth of endothelial cells, and inhibiting their apoptosis [3]. The expression of this protein is stimulated both by a variety of proangiogenic factors (EGF, PDGF, FGF, IL-1β) and by the environmental conditions of the cell (pressure and oxygen concentration, pH).Estimation of serum concentration VEGF-Ain the group of children with white type congenital heart disease was significantly increased. Thus, the synthesis of the studied growth factor increased 4 times, with an average value135.81±5.47 pg/ml, ranging from 94.8 to 177.2 pg/ml against the normative indicators of healthy babies, which averaged 34.01±1.42 pg/ml (P<0.001) (Fig. 2).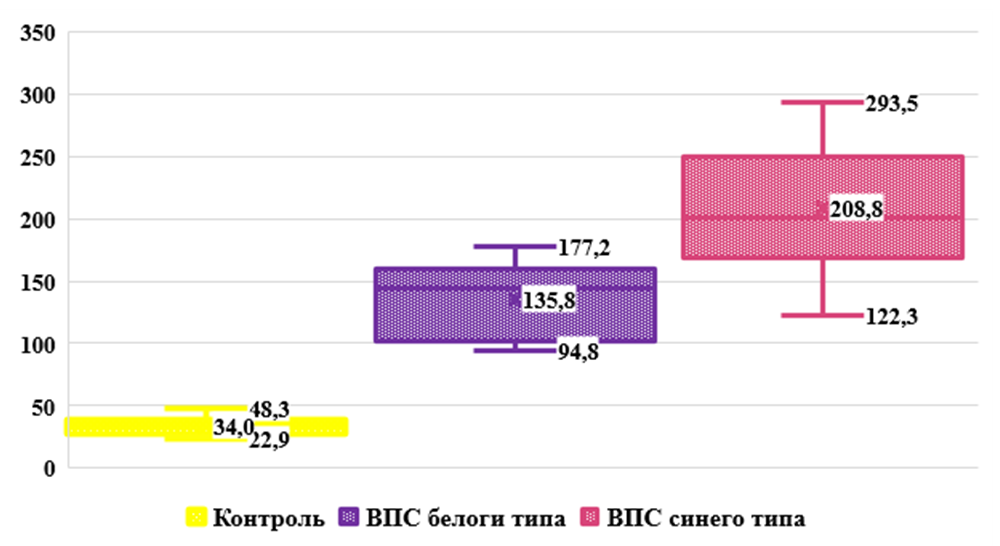 | Figure 2. Concentration VEGF-A in peripheral blood serum in the examined children in comparison. Note: * - significant compared with the data of the control group (* - P<0.05, ** - P<0.01, *** - P<0.001) |
In our opinion, the increased concentration VEGF-Ain the blood serum with congenital heart disease in the group of children without cyanosis may be due to factors such as hyperactivity of endothelial cells, angiogenesis, and vascular remodeling. These changes may cause increased production and release VEGF-Ain an attempt to restore normal vascular structure and function.According to the results obtained, shown in Fig. 2. in the main group of babies with blue type congenital heart disease, the serum level of VEGF-A was significantly increased. So the synthesis of the studied growth factor increased 6.1 times, with an average value 208.80±9.92 pg/ml, ranging from 122.3 to 293.5 pg/ml against the normative indicators of healthy babies, which averaged 34.01±1.42 pg/ml (P<0.001).The results obtained allow us to assume that the body of children with cyanosis “switches on” adaptation mechanisms to such processes as chronic hypoxia, inflammatory processes (in the form of a reaction to infections, tissue damage or other pathological conditions), trauma and tissue damage (to maintain remodeling processes and angiogenesis for tissue healing and repair) aimed at ensuring adequate blood supply to damaged tissues.TGF-beta (transforming growth factor beta/TGF-β) is a type of cytokine that controls proliferation, cell differentiation and other functions in most cells [15].TGF-β is a factor synthesized in a wide variety of tissues. It acts synergistically with TGF-alpha to cause phenotypic transformation and can also act as a negative autocrine growth factor. TGF-β has diverse functions in the body. When affecting the immune system, inhibitory effects predominate. In general, TGF-β plays an important role in the regulation of various processes in the body, including the immune response, tissue remodeling, wound healing and anabolic processes [4,5].Analysis of the obtained results shown in Fig. 3. revealed that the level of TGF-β is high in the group of children with white type CHD. Thus, the serum concentration of the studied growth factor in the main group of children was increased by 3.9 times, with an average value of 182.7 ± 7.31 pg/ml, with an individual range from 112.5 to 251.5 pg/ml, while in in the group of practically healthy children, this indicator was 47.42±2.21 pg/ml (P<0.001).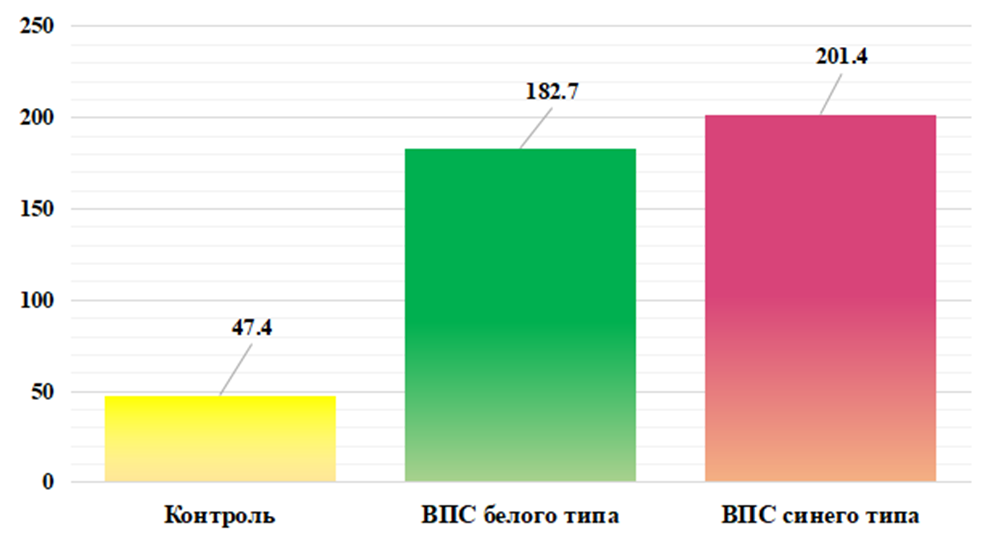 | Figure 3. Cserum content TGF-β in the examined children in comparison. Note: * - significant compared with the data of the control group (* - P<0.05, ** - P<0.01, *** - P<0.001) |
Our results likely indicate that elevated serum TGF-β levels in non-cyanotic congenital heart disease may be related to cardiac workload, inflammation and tissue remodeling, as well as the role of TGF-β in tissue development and differentiation. However, it should be noted that each individual case may be different, and additional studies may be necessary to more accurately determine the cause of elevated TGF-β levels.Evaluation of the results shown in Fig. 3. revealed significantly significant TGF-β levels in the group of children with blue type congenital heart disease. Thus, the serum concentration of the studied growth factor in the main group of children was increased by 4.2 times, with an average value of 201.45 ± 6.69 pg/ml, with an individual range from 125.3 to 255.1 pg/ml, whereas in the group In practically healthy children, this indicator was 47.42±2.21 pg/ml (P<0.001).Based on our results, we suggest that excess TGF-β in the blood can have both protective and destructive effects on the body, depending on the context and duration of its action. A strong increase in the level of TGF-β in the blood serum during congenital heart disease with cyanosis may be due to factors such as: hypoxia and ischemia in response to tissue damage and the body’s attempt to adapt to a changed environment; inflammation and tissue remodeling, mechanical factors, as with congenital heart defects there may be increased stress on the heart and blood vessels, which can activate the production of TGF-β in response to mechanical stress.Thus, in the course of immunological studies, it was revealed that hypoxia and ischemia in congenital heart disease, both with and without cyanosis, can stimulate an increase in the production of various cytokines and growth factors in response to damage and stress in tissues. In turn, congenital heart disease and hypoxia can cause remodeling cardiac and vascular tissue. This process involves the activation of bFGF, VEGF-A and TGF-β, which play an important role in tissue regeneration and remodeling.
4. Conclusions
Established immunological changes cause cascade reactions in the body in order to adapt to the lack of oxygen and blood supply. Activation of cells and tissues to cope with damage and stress leads to an increase in the production of various biologically active substances. Further research and clinical observations are needed to more accurately understand the role of these mediators in the pathology of various types of congenital heart disease.
References
| [1] | Belozerov, Yu.M. Pediatric cardiology / Yu.M. Belozerov. – M., 2004. – P. 9-221. |
| [2] | Boqueria, L.A. Cardiovascular surgery. 2001. Diseases and congenital anomalies of the circulatory system / L.A. Boqueria, R.G. Gudkova. – M., 2002. – 348 p. |
| [3] | Zakirova N.E., Zakirova A.N. The role of immunoinflammatory reactions and endothelial dysfunction in myocardial remodeling and progression of coronary artery disease. Rational Pharmacotherapy in Cardiology. 2014; 10(5): 488-94]. DOI:10.20996/1819-6446-2014-10-5-488-494. |
| [4] | Korzhenevskaya K. V., Gavrisheva N. A., Panov A. V. et al. Transforming growth factor-β1 in various clinical courses of coronary heart disease after coronary artery bypass surgery. Medical Immunology. 2010; 12(6): 521-8. |
| [5] | Moiseeva O.M., Shlyakhto E.V. Transforming growth factor and leukocyte activation markers in hypertension. Arterial hypertension. 2003; 9(1): 14-6]. DOI:10.18705/1607-419X-2003-9-1-14-16. |
| [6] | Podzolkov V.P., Shvedunova V.N. Congenital heart defects. RMJ. 2001; 10: 430. |
| [7] | The role of VEGF in the development of neoplastic angiogenesis / V. P. Chekhonin [et al.] // Vestn. RAMS. 2012. No. 2. P. 23-34. |
| [8] | Sharykin, A.S. Perinatal cardiology: director. for pediatricians, obstetricians, neonatologists / A.S. Sharykin. M., 2007. 264 p.Ardizzone A., Scuderi S.A., Giuffrida D., Colarossi C., Puglisi C., Campolo M., Cuzzocrea S., Esposito E., Paterniti I. Role of Fibroblast Growth Factors Receptors (FGFRs) in Brain Tumors, Focus on Astrocytoma and Glioblastoma. Cancers. 2020; 12: 3825. doi: 10.3390/cancers12123825. |
| [9] | Arita S., Kikkawa F., Kajiyama H., Shibata K., Kawai M., Mizuno K., Nagasaka T., Ino K., Nomura S. Prognostic importance of vascular endothelial growth factor and its receptors in the uterine sarcoma. Int. J. Gynecol. Cancer, 2005, Vol. 15, no. 2, pp. 329-336. |
| [10] | Ferrara N. Vascular endothelial growth factor: basic science and clinical progress / N. Ferrara // Endocr. Rev. 2004. Vol. 25. P. 581–611. |
| [11] | Flavell SJ, Hou TZ, Lax S, Filer AD, Salmon M, Buckley CD. Fibroblasts as novel therapeutic targets in chronic inflammation. British Journal of Pharmacology. 2008; 153(S1): S241–S246. DOI: http://dx.doi.org/10.1038/sj.bjp.0707487. |
| [12] | Hartlapp I, Abe R, Saeed RW, Peng T, Voelter W, Bucala R, et al. Fibrocytes induce an angiogenic phenotype in cultured endothelial cells and promote angiogenesis in vivo. FASEB journal. 2001 Oct; 15(12): 2215–2224. DOI: http://dx.doi.org/10.1096/fj.01-0049com. |
| [13] | Keeley EC, Mehrad B, Strieter RM. Fibrocytes: Bringing new insights into mechanisms of inflammation and fibrosis. The international journal of biochemistry & cell biology. 2010 Apr; 42(4): 535–542. DOI: http://dx.doi.org/10.1016/j.biocel.2009.10.014. |
| [14] | Verrecchia F., Mauviel A. Transfoming growth factor-beta and fibrosis. World J Gastroenterol. 2007; 47(8): 13-18. DOI:10.3748/wjg. v13.i22.3056. |




 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML
