-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2024; 14(2): 484-490
doi:10.5923/j.ajmms.20241402.66
Received: Feb. 3, 2024; Accepted: Feb. 19, 2024; Published: Feb. 24, 2024

Cardiovascular Complications in Patients with COVID-19 Complicated by Viral Pneumonia
Tashkenbaeva Eleonora Negmatovna, Togaeva Barchinoy Musoqulovna, Abdieva Gulnora Alievna, Haydarova Dilrabo Davronovna
Department of Internal Diseases and Cardiology № 2, Samarkand State Medical University, Samarkand, Uzbekistan
Correspondence to: Abdieva Gulnora Alievna, Department of Internal Diseases and Cardiology № 2, Samarkand State Medical University, Samarkand, Uzbekistan.
| Email: |  |
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

COVID-19 (Coronavirus Disease 2019) causes significant lung damage, including pneumonia and acute respiratory distress syndrome (ARDS). At the same time, researchers have observed many extrapulmonary manifestations of this formidable infectious disease. Accumulating clinical experience and emerging research suggest that in addition to the respiratory system, cardiovascular, hematological, renal, gastrointestinal and hepatobiliary, endocrinological, neurological, ophthalmic and dermatological systems may be affected. To identify the incidence of cardiovascular complications in patients with COVID-19 complicated by viral pneumonia, to compare the results obtained with the data of literature sources and to substantiate their pathogenetic occurrence. The object of the study was 70 patients with viral pneumonia caused by COVID-19, who received treatment in a COVID specialized center. The subject of the study is the blood and blood serum of patients with coronary artery disease for the quantitative determination of the main biochemical parameters (lipid spectrum). The novel coronavirus infection affects not only the respiratory system, but also has a significant impact on the state of the cardiovascular system, both due to the patient's immune response and due to the probable cytopathic effect of the virus. This is evidenced by the high incidence of CVS complications, the most common of which were valve regurgitation, including concomitant cardiac arrhythmias, of which supraventricular arrhythmias were the most common.
Keywords: COVID-19, Arterial hypertension, Blood pressure, Comorbidity, Cardiovascular complications
Cite this paper: Tashkenbaeva Eleonora Negmatovna, Togaeva Barchinoy Musoqulovna, Abdieva Gulnora Alievna, Haydarova Dilrabo Davronovna, Cardiovascular Complications in Patients with COVID-19 Complicated by Viral Pneumonia, American Journal of Medicine and Medical Sciences, Vol. 14 No. 2, 2024, pp. 484-490. doi: 10.5923/j.ajmms.20241402.66.
Article Outline
1. Introduction
- As you know, COVID-19 (Coronavirus Disease 2019) causes significant lung damage, including pneumonia and acute respiratory distress syndrome (ARDS). At the same time, researchers have observed many extrapulmonary manifestations of this formidable infectious disease. Accumulating clinical experience and emerging research suggest that in addition to the respiratory system, cardiovascular, hematological, renal [1], gastrointestinal and hepatobiliary [2,3], endocrinological, neurological [1], ophthalmic and dermatological systems may be affected. This pathology may reflect either extrapulmonary spread and replication of SARS-CoV-2, as observed for other zoonotic coronaviruses [22], or widespread immunopathological consequences of the disease. To give an idea of these extrapulmonary manifestations, including cardiovascular system (CVS), it is necessary to consider the crucial role of clinical and pathogenetic aspects of the development of multiple organ lesions in COVID-19 involving the cardiovascular system. In the early stages of COVID, the lungs are the main organ affected. The COVID-19 pathogen, SARS-COV-2, uses the angiotensin-converting enzyme 2 (ACE2) receptor, which is abundant in the lower respiratory tract, to enter cells. Very importantly, ACE2 is also expressed in the heart, intestinal epithelium [3,4], vascular endothelium, and kidneys [1], making all these organs potential targets [9]. SARS-CoV-2 is a spherical particle with a diameter of approximately 120 nm containing a single-stranded RNA genome. It is classified as a beta coronavirus (β-CoV) [lineage B] and is the seventh coronavirus to infect humans, after 2 α-CoV (HCoV-229E and HKU-NL63) and 4 β CoV (HCoV-OC43 [lineage A), HCoV-HKU1 [lineage A], severe acute respiratory syndrome SARS-CoV [lineage B], and Middle East respiratory syndrome MERS-CoV [lineage C]) [12,13,18]. Structural proteins of SARSCoV-2 include S-proteins or "spike proteins", membrane protein, envelope protein, and nucleocapsid. The presence of spike-shaped S-proteins on electron microscopic imaging shows a "halo" or "crown" around the virus, which is why the virus was given the appropriate name. The S-protein plays an important role in the attachment, fusion and entry of the virus into cells, which allows it to be considered as a possible target to produce antibodies and vaccines. The angiotensin-converting enzyme 2 receptor (ACE2) is the main receptor for the spike-shaped S-protein of the virus and determines the infectivity of the pathogen [32]. After initial infection, the development of acute disease can be divided into three distinct phases (early phase of infection, pulmonary phase, and hyperinflammatory phase) with significant overlap [7,14]. The hyperinflammatory stage is characterized by a cytokine storm leading to immune-mediated damage to distant organs [4]. Studies have demonstrated significant increases in inflammatory markers, including interleukin (IL)-6, -2, -7, tumor necrosis factor (TNF)-α, interferon-inducible protein (IP)-10, chemoattractant monocyte protein (MCP). -1, macrophage inflammatory protein (MIP)-1α, granulocyte colony stimulating factor (G-CSF), C-reactive protein (CRP), procalcitonin, and ferritin [25,60]. There are several mechanisms of cardiac damage, including direct myocardial damage by the virus itself, hypoxic damage mediated by respiratory failure, indirect cytokine-mediated damage secondary to the systemic inflammatory response, myocardial infarction (MI) due to plaque rupture secondary to systemic inflammation [62]. Direct damage to the heart mediated by the ACE2 receptor also remains a possibility. ACE2 receptors are expressed in cardiac pericytes and endothelial cells, and experimental data in animals suggest that their direct dysfunction secondary to viral infection or secondary inflammation may cause MI [16,64]. Increases in cardiac biomarkers, including troponin T, have been shown to be linearly correlated with inflammatory markers, indicating that myocardial damage is likely related to underlying inflammation [27].Objective: to identify the incidence of cardiovascular complications in patients with COVID-19 complicated by viral pneumonia, to compare the results obtained with the data of literature sources and to substantiate their pathogenetic occurrence.
2. Materials and Methods of Research
- 70 patients with viral pneumonia caused by COVID-19. The comorbid background of patients, the data of routine methods of laboratory (complete blood count (CBC), troponin test: qualitative and quantitative) and instrumental (electrocardiography (ECG), echocardiography (echocardiography)) diagnostics were studied. Indicators of descriptive statistics were calculated: for indicators with a normal distribution, the results are presented in the form of an arithmetic mean, standard deviation, in other cases - in the form of median and interquartile range, categorical variables were presented in the form of quantity and percentage. The normality of the distribution was tested using the Shapiro-Wilk test. Statistical significance analyses were performed using the unpaired t-test and the Mann-Whitney U test for measures with a non-normal distribution. The critical value of the level of statistical significance was assumed to be p≤0.05. In the discussion, an analysis of the literature in the databases eLIBRARY.ru, PubMed, GoogleScholar, WebofScience for recent years, mainly for 2020-2021, was carried out to compare the results obtained.
3. Results and Discussion
- A total of 70 patients with viral pneumonia were examined. Among them, 38 (54.3%) were men and 32 women (45.7%), the mean age was 54 years with an interquartile range from 49.75 to 71.25 (Fig. 1). The sample was dominated by patients over the age of 60 years among both men and women.
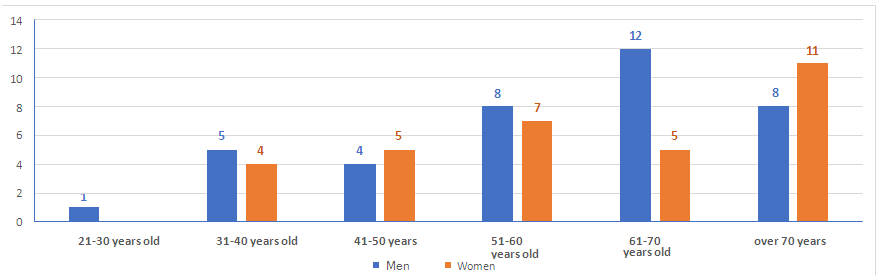 | Figure 1. Distribution of patients according to gender and age |
4. Conclusions
- The novel coronavirus infection affects not only the respiratory system, but also has a significant impact on the state of the cardiovascular system, both due to the patient's immune response and due to the probable cytopathic effect of the virus. This is evidenced by the high incidence of CVS complications, the most common of which were valve regurgitation, including concomitant cardiac arrhythmias, of which supraventricular arrhythmias were the most common.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML