-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2024; 14(2): 481-483
doi:10.5923/j.ajmms.20241402.65
Received: Feb. 5, 2024; Accepted: Feb. 17, 2024; Published: Feb. 24, 2024

Use of Non-Invasive Methods of Diagnosing Liver Fibrosis in Patients with Chronic Virus Hepatitis C
Akhmedova Nilufar Sharipovna, Yuldashev Jasur Azatovich
Bukhara State Medical Institute, Bukhara, Uzbekistan
Correspondence to: Yuldashev Jasur Azatovich, Bukhara State Medical Institute, Bukhara, Uzbekistan.
| Email: |  |
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The high incidence and prevalence of chronic viral hepatitis C is an urgent problem for world medicine. The prevalence of the disease among the working population means its negative impact on the country's economy and social spheres. Fibrosis, as a typical pathophysiological process, is the consequence of a large number of chronic diseases and is considered the main pathogenetic mechanism of the final stages of diseases and death. In the case of non-invasive diagnosis of liver fibrosis, when it is not possible to perform elastometry, it is appropriate to use scales for determining liver fibrosis.
Keywords: Chronic viral hepatitis, Elastometry, Liver fibrosis, Polymerase chain reaction analysis, Antiviral therapy
Cite this paper: Akhmedova Nilufar Sharipovna, Yuldashev Jasur Azatovich, Use of Non-Invasive Methods of Diagnosing Liver Fibrosis in Patients with Chronic Virus Hepatitis C, American Journal of Medicine and Medical Sciences, Vol. 14 No. 2, 2024, pp. 481-483. doi: 10.5923/j.ajmms.20241402.65.
1. Introduction
- Today, the number of people infected with chronic viral hepatitis C in the world is about 75 million, and the rate of death from this disease is about 5.5 million [2].Mortality in viral hepatitis50-60% occur as a result of the terminal stage of liver fibrosis (cirrhosis).The high incidence and prevalence of Chronic viral hepatitis C is an urgent problem for world medicine. The prevalence of the disease among the working population means its negative impact on the country's economy and social spheres.Because the majority of patients with Chronic viral hepatitis C are unaware that they have the virus, any patient with known risk factors should be screened. Active intervention is needed to test for HVC antibodies in patients at high risk of Chronic viral hepatitis C infection. In particular, screening should be carried out in risk groups with a high probability of parenteral transmission of the virus [4,6].The pathogenesis of the development of Chronic viral hepatitis C has not been fully studied to date. In particular, extra-hepatic complications of the disease are common, which accelerates the progression of the main disease, cirrhosis and fibrosis of the liver, and leads to an increase in the rate of lethality in patients. [1,3,4].Problems related to screening, diagnosis and treatment must be solved in each country, especially at the level of local health institutions. Patients with any risk factor for chronic viral hepatitis should be screened for anti-HCV antibodies by their family physician. "Latent patients" (patients previously positive for HCV antibodies and PCR, but never treated) are an important group that should be actively sought for intervention and should be recommended and treated with modern laboratory tests [4].The long symptomless course of Chronic viral hepatitis C, the fact that the diagnosis is often complicated by hepatocellular carcinoma in the terminal stages of the disease, requires a pathogenetic approach to the diagnosis of this disease. The fibrosis process underlies the progression and complications of Chronic viral hepatitis C.Liver fibrosis is the growth or accumulation of connective tissue in the liver as a result of chronic processes of various etiologies [5].Stellate cells play an important role in the pathogenesis of connective tissue growth in the liver. Factors that stimulate the growth of connective tissue: collagen, glycocoprotein, glycosamine and protein receptors are also important in this process.Fibrosis, as a typical pathophysiological process, is the consequence of a large number of chronic diseases, and is considered the final stages of diseases and the main pathogenetic mechanism of death [3-6].This condition is typical for chronic liver diseases, including Chronic viral hepatitis C [1-3]. Liver fibrosis progressing to liver cirrhosis is the most important and urgent problem of chronic viral hepatitis.Based on this, extensive research is being conducted today to identify new histological, serological and genetic markers of liver fibrosis. The main goal of these studies is to solve the problems of pathogenetic therapy [4].The purpose of the research work a comparative study of the diagnostic and prognostic value of non-invasive methods of assessing liver fibrosis.
2. Material and Methods
- 116 patients diagnosed with chronic viral hepatitis C who applied to the Khorezm Regional Infectious Diseases Hospital and received inpatient treatment were involved. 46 patients were initially diagnosed and did not receive etiotropic treatment, and the second group included 70 patients receiving combined antiviral therapy. Instrumental and laboratory aspects of liver fibrosis diagnosis in both groups were compared. All patients were diagnosed with Chronic viral hepatitis C by IFA and PCR. In evaluating the fibrosis process, elastometry (Fibroscan) from instrumental diagnostic methods and liver fibrosis index (JFI) from non-invasive methods, the most widely used APRI, FIB-4 scales were used. Statistical analyzes were conducted in Microsoft Excel based on Student's and Pearson's criteria. The principle of evidence-based medicine was used in the organization and conduct of the study.
3. Analysis of Results
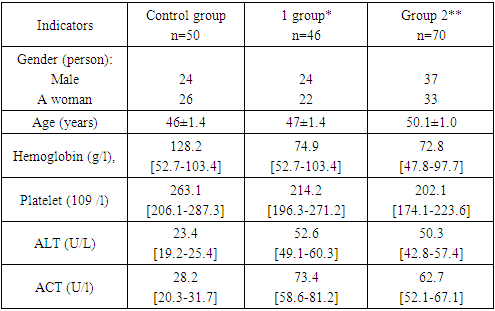
- 61 (52.6%) men and 55 (47.4%) women were included in the study, and the patients were divided into 2 groups. The first group consisted of 46 patients with primary diagnosis of chronic viral hepatitis C, the average age was 47±1.2 years. The second group consisted of 70 patients with chronic viral hepatitis C receiving antiviral therapy, the average age was 50.1±1.7, and the duration of the disease was 6.7±0.7 years. Secondpatients in the group received combined antiviral therapy against the hepatitis C virus. "Triple therapy" (protease inhibitor + interferon + ribavarin) is recommended for antiviral therapy.General information about the examinations and their results in the patients included in the study is presented in Table 1.The table shows the average indicators of clinical and laboratory instrumental examinations, meeting rates and percentages in both groups of patients, which are important in assessing the level of liver fibrosis development.
|
|
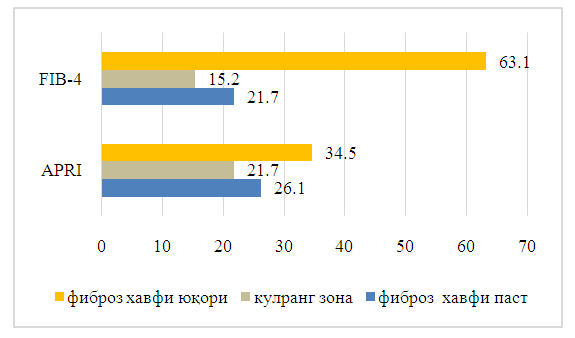 | Figure 1. Results of liver fibrosis using the APRI and FIB-4 scales |
4. Conclusions
- In conclusion, it can be said that when it is not possible to perform elastometry in the non-invasive diagnosis of liver fibrosis, it is appropriate to use scales for determining liver fibrosis.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML