-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2024; 14(2): 466-470
doi:10.5923/j.ajmms.20241402.61
Received: Feb. 2, 2024; Accepted: Feb. 21, 2024; Published: Feb. 22, 2024

Pathogenetic Basis for the Development of Lactase Enzyme Deficiency
Azimova Sevara Bahodirovna1, Samieva Gulnoza Utkurovna2, Khidirova Sojida Khusniddin Kizi3
1Doctor of Medical Sciences, Associate professor of the Department of Normal and Pathological Physiology, Tashkent Medical Academy, Uzbekistan
2Doctor of Medical Sciences, Head of the Department of Normal and Pathological Physiology, Samarkand State Medical University, Uzbekistan
3PhD Student, Department of Normal and Pathological Physiology, Samarkand State Medical University, Uzbekistan
Correspondence to: Khidirova Sojida Khusniddin Kizi, PhD Student, Department of Normal and Pathological Physiology, Samarkand State Medical University, Uzbekistan.
| Email: |  |
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Lactase deficiency (LY) is a disorder of the functioning of the congenital or acquired digestive system that is accompanied by a violation of the absorption of lactose (milk shikari), especially in the small intestine. In the Normal state, milk sugar is broken down into monosaccharides glucose and galactose by the action of the enzyme lactase in the small intestine cavity and is actively transported in the small intestine. Clinical manifestations of enzyme deficiency are determined by lactase deficiency. The enzyme mainly activates in the vortices of the apical part of the enterocytes of the small intestine. Low enzyme activity increases the risk of developing malabsorption syndrome. Patalogy, which is common all over the world today, is a disease in which a violation of the digestion and absorption process in the intestines accompanies lactase deficiency. The relevance of studying this disease is that the young organism that is, newborns feed mainly on breast milk and dairy products and adapt to the external environment, in which the role of lactase is played. The article provides a brief overview of enzyme deficient, its ethylogical factors, and novel treatments.
Keywords: Lactose, Lactase deficiency, Ethylogical factors, Genetic factors, Pathogenesis
Cite this paper: Azimova Sevara Bahodirovna, Samieva Gulnoza Utkurovna, Khidirova Sojida Khusniddin Kizi, Pathogenetic Basis for the Development of Lactase Enzyme Deficiency, American Journal of Medicine and Medical Sciences, Vol. 14 No. 2, 2024, pp. 466-470. doi: 10.5923/j.ajmms.20241402.61.
1. Introduction
- LY lactase deficiency is a pathology of the small intestine characterized by the development of a maldigestive syndrome caused by the absence or insufficient activity of the lactase enzyme. Lactase deficiency was first described by Hippocrates in the 400s BC, but its clinical signs have only been recognized in applied medicine in the last 50 years. Lactase deficiency is the most common form of lactase metabolism disorder, affecting nearly 4 billion people worldwide. The amount of lactose that causes clinical symptoms varies with the degree of lactase deficiency. The greatest importance of the problem occurs in children at an early age, since during this period dairy products prevail in the diet. [1] children most consume cow's milk and dairy products, but at the same time are the most common cause of food intolerance. Food intolerance can be caused by any component of milk, and it develops both immune and non-immune forms. The most common cases in clinical practice are lactase deficiency and intolerance to cow's milk proteins. In this regard, knowledge of the features of pathogenesis, etiological factors and modern approaches to the diagnosis of lactase deficiency in children is necessary in the daily work of a pediatrician.
2. Methodology
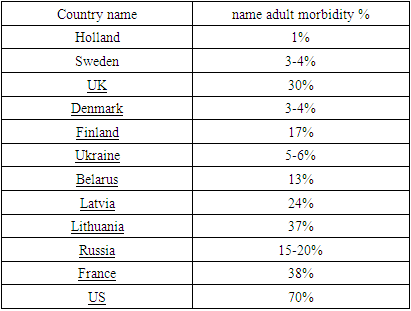
- According to its etiology, lactase deficiency is divided into primary or secondary. Primary lactase deficiency is caused by genetic deficiency of the enzyme lactase. Secondary lactase deficiency is caused by a decrease in lactase activity in the mucous membrane of the small intestine as a result of damage to the endocrine system and occurs in cases of extensive resection of the small intestine, parasitic diseases and previous bacterial infections of the intestine. For many years, it has been believed that secondary lactase yachting, which develops in adults, is a natural condition associated with the complete loss of lactose activity and does not require special treatment. Secondary lactase deficiency occurs in 30-40% of cases in adults. In old age and old age, secondary lactase deficiency is a manifestation of age-related malformation, with a frequency of 70% [2].Basically, LY affects the population of Asia and Africa (90%), southern Europe (70%) and South America. Lactase deficiency is very rare in northern Europe and the United States [3,4,5,6,25].
|
3. Discussion
- The pathophysiological properties of this disease are such that in the small intestine, undigested lactose enters the large intestine in large quantities due to a decrease in the activity of the enzyme lactase in the brush border of enterocytes, where milk is under the influence of beta-galactosidases. acidic bacteria, it is converted into unbranched short-chain fatty acids (acetate, propionate, butyrate), carbon dioxide, methane, hydrogen and water [12]. Hydrogen is excreted in large quantities by the lungs, the hydrogen breath test for the diagnosis of hypolactasia is based on this. The spectrum of fatty acid formation depends on the type of diet of the child.Thus, breastfed children produce mainly acetate, while artificially fed children produce butyrate [13]. Butyrate plays an important role in starting the production of anti-inflammatory cytokines. The increase in lactose fermentation products leads to an increase in osmotic pressure, and today the inclusion of an undefined signaling mechanism that regulates the intracellular permeability of the intestinal epithelium causes osmotic diarrhea. When lactose is broken down by colon bacteria, toxic metabolites (ethanol, acetone and other ketones, diols such as butane-2,3-diol, acids and aldehydes, i.e. methylglioxal, acetaldehyde, protein toxins) are formed, altering cell metabolism.These toxins stimulate Ca2+-dependent signaling channels in bacteria (for example, methylglyoxal blocks bacterial growth for 5 hours), cause an imbalance of microflora competing for substrates, and disrupt the transmission of information in the bacterial-master cell system. Lactose can also have toxic effects when absorbed in an unfermented form [14,15].Currently, lactase activity has been shown to be primarily associated with the enzyme lactase-fluorinating hydrolase, the primary glycoprotein of the brush border membrane of enterocyte microvorsincs.This enzyme exhibits high lactase (beta-D-galactoside hydrolase) and fluorizine hydrolase activity (glycosyl N-acetylsfingosine glucohydrase), promoting the degradation of lactose and some other glucosides, such as fluorizine, which regulates the absorption of monosaccharides. Lactase is synthesized as a single-stranded pre/prolactase precursor composed of a sequence of 1927 amino acids (4). Lactase activity is associated with the Glu-1749 site. Subsequently, the enzyme undergoes a series of sequential changes in the endoplasmic reticulum (with proferment formation) and the Golji complex (with the formation of a 3-dimensional structure characteristic of the"mature" enzyme). The mature enzyme enterocyte is transported to the Gill border membrane. In this, the N end of the molecule is located outside the cell membrane, and the C end is located in the cytosol. The active enzyme has 2 catalytic fields.The clinical manifestations of lactase deficiency are very diverse, depending on the level of enzyme activity, the amount of lactose in the diet, the individual sensitivity of the intestine and the characteristics of intestinal biocenosis. The presence and types of clinical manifestations of lactose intolerance are often not associated with the degree of enzyme reduction. Lactose intolerance depends not only on the level and activity of the enzyme, but also on the number of lactose-fermenting bacteria. Today, many factors are known that affect lactase activity.Epidermal growth factor-increases the production of brush border enzymes and is important in intrauterine development of the fetus. In addition, it has been proven that breast milk contains a large amount of epidermal growth factor that affects lactose intolerance. Of great importance for lactase activity are insulin, thyroid hormones, glucocorticoids and the state of the autonomic nervous system. The appearance of clinical signs of lactase deficiency also depends on the composition of the diet: the consumption of lactose along with fats helps to reduce the manifestation of intolerance [16].
4. Results
- Many factors affect the functional state of the mucous membrane of the small intestine and lactase:1. Gestation period. In ontogenesis, late maturation of the enzyme leads to temporary LY in premature infants. However, bacterial fermentation of lactose during breastfeeding leads to the lack of clinical significance of LY in most premature babies [17].2. Genetic factors. The gene encoding lactase synthesis is secreted at the 2q21 locus in the long arm of chromosome 2. The gene encoding lactase synthesis in the body, the LCT gene, is located on chromosome 2q21.Lactose tolerance is primarily determined by the presence of the activity stability gene (LCT). Peoples who have historically led a nomadic lifestyle and engaged in animal husbandry have a significantly higher frequency of the gene for the stability of lactase activity, and good milk tolerance mutations affecting hypolactasia have now been identified. Its genetic basis is the 9 single nucleotide polymorphisms (English single nucleotide polymorphism, SNP) in the regulatory element. SNPs are located in the intron of the MCM6 gene at a distance of several kilobase pairs from the 5/ end of the LCT, i.e. above the starting point of transcription. The location of nucleotides determines the phenotype (replacement of cytosine (c) thymine (T) and arginine (a) by guanine (G) (G/A-22018)) at position 13910 (c/T-13910).C/T locus in adults <<13910 T allele C in comparison to the allele (2-22%), the higher order of magnitude of mRNA synthesis (LCT gene 78-98% of total mRNA) is determined. The genotype corresponds to a near-complete absence of C/C <13910 lactase. The C / T-13910 genotype is associated with decreased lactase levels, which is sufficient for normal digestion. T / T genotype < 13910 indicates high enzyme activity [18].3. Hormonal factors. In many cases, data on the effect of hormones obtained in animals on lactase activity suggest that hormones associated with "stress hormones" play the greatest role in lactase activation. In addition, the effect is manifested only during breastfeeding, in adulthood it is practically absent [20]. Glucocorticoids reduce the enterocyte division cycle, increase lactase activity during pregnancy, and accelerate the decrease in its activity when switching to an adult type of diet in animals [19]. Somatotropic hormone has trophic effects on enterocyte by means of insulin-like growth factor. Insulin increases enzyme activity during pregnancy.4. Autonomic nervous system. It is known that in children under 3-4 years old, the influence of the sympathetic nervous system prevails, which weakens the mobility of the gastrointestinal tract, digestive processes slow down, liver cells and adipose tissue release more glucose, etc. Fatty acids enter the blood and the pancreas produces less insulin. The parasympathetic system increases blood flow to the digestive system, accelerates the movement of food through the intestines, and enhances the secretion of digestive enzymes [20].5. Protein growth factors accelerate the division and maturation of enterocytes. The Epidermal growth factor affects the proliferation of intestinal epithelial cells, primarily crypts, The Shape of the brush border membrane (due to changes in its fluid), and the expression of disaccharidases in it. Among trophic factors, this is the most popular. It has the ability to act independently of hormones in the enterocyte, has a trophic effect and affects the growth of the cell. It should also be noted that a large amount of this factor is present in breast milk, therefore, a nursing baby is better adapted to a decrease in enzyme activity. Insulin-like growth factors 1 and 2 are synergists of the previous factor [21].6. Space factors. The presence of food in the intestinal cavity is the strongest trophic factor for its mucosa [19]. Breast milk contains substances that directly affect the reproduction of the mucous membrane. These are amino acids, polyamines, nucleotides.7. Enterocyte damage factors reduce lactose fermentation due to direct injury and increased intestinal transit rate; these are infectious, toxic, allergic effects on the body [22].Many laboratory diagnostic methods are non-informative or invasive and expensive, so these methods are additional research methods to carefully obtain Anamnesis, correctly interpret clinical symptoms, and help the doctor make the correct diagnosis [23]. When examining the clinical picture of ly, it became known that patients often complain of painful pain in the epigastral area after eating, itching with air, nausea, flatulence, diarrhea, decreased appetite, low weight, avitaminosis, tumors in the abdominal area and stool disorders. Since the diagnosis of each patient includes other diseases (gastritis, bacterial growth syndrome, intestinal syndrome of unknown etiology, etc.) in addition to LY, some clinical manifestations may be associated with them, and not with LY. In addition, food allergies have often been found to be associated with LY. In physical examination of the patient, in most cases, there is white Carache and tooth marks on the tongue, pain in the epigastrium and the presence of edema in palpation of the abdomen.Currently, the subject of scientific research is, on the one hand, the prebiotic potential of lactose, resistance to the development of inflammatory bowel diseases, on the other hand, the elderly cortical cataract caused by galactose in people who tolerate milk sugar, as well as lactose. protection against malaria, atherosclerosis and ovarian carcinoma.
5. Conclusions
- Thus, given the high prevalence of hypolactasia and the polymorphism of its clinical manifestations, we can recommend expanding the guidelines for diagnostic measures that determine the presence of LY. At the same time, further research on the degree of attachment of individual signs or symptom complexes with the presence of LY is needed to determine specific indicators. Various metabolic disorders require the development of new and existing diagnostic methods. The LCT C/C-13910 genotype that detects lactose intolerance is higher in children with atopic diseases (bronchial asthma, hay fever, atopic dermatitis, cow's milk protein allergy) compared to the general population. Lack of lactase stability is not a disease, but a simple human phenotype formed during evolution. However, extending the lactotrophic diet to periods of human life accompanied by suppression of lactase production requires dietary changes and, possibly, drug adjustments for individuals with the LCT C/T genotype. Starting treatment for LY should begin with prescribing a lactose-free diet, and after providing clinical remission, the lactose load should be gradually increased to an individual limit dose. The appropriateness and tolerance of the lactose dose can be estimated based on hydrogen breath test data. To increase the limit dose of lactose and the safety of treatment, we recommend the use of exogenous lactose preparations, in particular those produced from Aspergillus oryzae.Treatment of primary LY is carried out throughout life, while secondary LY is carried out only until the production of lactase is restored in the intestinal mucosa. The recipe for calcium and vitamins, in our opinion, should be carried out depending on the presence of their deficiency.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML