-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2024; 14(2): 402-408
doi:10.5923/j.ajmms.20241402.51
Received: Jan. 17, 2024; Accepted: Feb. 12, 2024; Published: Feb. 20, 2024

Role of Neurospecific Proteins and Indicators Magnetic Resonance Spectroscopy in Early Diagnostics of Cognitive Deficities in Children and Adolescents with Type 1 Diabetes
Alidjanova Durdona1, Sultanova Shakhrizada2, Pirnazarov Maruf3, Abduraimov Abbos3
1Tashkent Pediatric Medical Institute, Tashkent, Uzbekistan
2Republican Specialized Scientific and Practical Medical Center for Endocrinology named after Academician Y.N. Turakulova, Tashkent, Uzbekistan
3Republican Scientific and Practical Center for Sports Medicine under the National Olympic Committee of the Republic of Uzbekistan, Tashkent, Uzbekistan
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

This article contains our own results of analysis of neuropsychological, laboratory and neuroimaging examination methods related to the problem of cerebral complications in type 1 diabetes in children. Data from our own studies of psychological testing (MoCa test), Spielberger-Khanin methods for assessing situational (ST) and personal (PT) anxiety are presented. In addition, blood levels of S-100 protein and neuron-specific enolase (NSE) were demonstrated to study the pathogenetic and diagnostic roles of neuropeptides and major brain metabolites. Using proton magnetic resonance spectroscopy, the concentrations of brain metabolites N-acetylaspartate (NAA), choline (Cho) and creatine (Cr), and their ratios (NAA / Cr; Cho / Cr; Cho / NAA) were determined. According to the results obtained, we can say that the proposed set of diagnostic measures using modern methods of brain research in the early diagnosis of manifestations of cognitive and emotional disorders in this pathology is reasonable and appropriate, since it is possible to trace changes in brain tissue in the form of microstructural damage and visualize deviations in brain metabolism leading to cerebral dysfunction even at the preclinical stage.
Keywords: Cognitive deficit, Children and adolescents, Diabetes mellitus, Neurospecific proteins, Proton magnetic resonance spectroscopy
Cite this paper: Alidjanova Durdona, Sultanova Shakhrizada, Pirnazarov Maruf, Abduraimov Abbos, Role of Neurospecific Proteins and Indicators Magnetic Resonance Spectroscopy in Early Diagnostics of Cognitive Deficities in Children and Adolescents with Type 1 Diabetes, American Journal of Medicine and Medical Sciences, Vol. 14 No. 2, 2024, pp. 402-408. doi: 10.5923/j.ajmms.20241402.51.
1. Introduction
- Diabetes mellitus (DM) is considered to be an endocrine autoimmune pathology that develops as a result of an absolute or relative deficiency of insulin caused by the destruction of beta cells of the pancreas. At the present stage, diabetes is a serious medical and social problem, as it remains one of the leading causes of the development of chronic complications with the occurrence of early disability in patients. Diabetes mellitus is characterized by persistent hyperglycemia, which is accompanied by abnormalities of various organs and systems [1]. Among the variety of complications of type 1 diabetes in children, a special place is given to changes in the brain, mainly manifested by cognitive impairment (CI). Close attention to cognitive activity in diabetes mellitus is associated not only with the normal daily functioning and social adaptation of the child, but also with regular adequate self-monitoring of glycemia, which directly affects the overall course of the underlying disease [2]. Currently, the problem of early diagnosis of cognitive deficits in patients with diabetes occupies one of the leading places in modern neuropathology [3]. In the diagnosis of disorders of higher cortical activity, for example, such as deficits in memory, attention, and thinking, methods of neuropsychological testing have a dominant position. However, here we have to take into account that these methods still provide a subjective assessment with the presence of both false positive and false negative results and are not able to offer complete reliable information on all criteria of cognitive deficit of interest [4]. Therefore, the issue of adequate and at the same time early diagnosis of these disorders with objective criteria that most accurately indicate the localization of the pathological focus causing cognitive changes is considered quite relevant not only for neurologists, but also for specialists in other areas of medicine. According to this, the primary task today is to identify the risks of developing cognitive dysfunctions long before their occurrence. The development and implementation of a clear algorithm for predicting cognitive deficit at the stage of the absence of its clinical signs can solve the problem and guide specialists in prescribing adequate preventive therapy, which in turn will contribute to the preservation of higher cortical functions at a satisfactory level for a sufficiently long time [5]. In this regard, in recent years, the interest of researchers in the biochemical components of cognitive impairment has increased significantly. Thus, much attention is paid to determining the content of various neurospecific proteins in the blood serum as markers of neurodegenerative and neuroinflammatory processes or, for example, using proton magnetic resonance spectroscopy to analyze the content of the main metabolites responsible for the structural and functional integrity of brain cells. Neurospecific proteins are tissue-specific for the nervous system and are histogenetically found in neurons and glial cells. In blood serum, the normal content of these proteins is considered to be low concentrations; this occurs due to the death of neurons, for natural reasons, as well as as a result of pathological changes. In this connection, an increase in the level of neurospecific proteins is considered in the format of markers of various pathological processes in the brain of adults and children, the most studied of which are epilepsy, head injury, diabetes mellitus, consequences of hypoxia, autism, Parkinson’s disease. [6,7]. Of the total number of known neurospecific proteins, the most studied are protein S-100 and neuron-specific enolase (NSE), which, according to scientists, are more informative about the state of the pathological process and can be useful in studying the pathogenesis of neurological dysfunction, including in children and adolescents with type 1 diabetes [8].The use in clinical practice of a method such as proton magnetic resonance spectroscopy (PMRS) has made it possible to intravitally and non-invasively assess metabolic changes occurring in the brain in various neurological pathologies. This neuroimaging technique is based on the so-called “chemical shift” of the resonant frequencies of various chemical compounds. To date, two methods of magnetic resonance (MR) spectroscopy have been developed, these are single-voxel and multivoxel spectroscopy. The first spectroscopy technique provides rapid analysis of the biochemical profile of a localized volume within a brain region of interest. The second method of MR spectroscopy is based on the use of so-called color mapping, which covers a fairly large area of the brain with a multi-voxel volume, allowing localization from 16 to 64 voxels simultaneously, including not only the area of the altered brain matter, but even the opposite hemisphere, and at different anatomical levels [9]. The main metabolites studied by MR spectroscopy are N-acetylaspartate (NAA), choline (Cho) and creatine (Cr), and in addition to assessing their absolute concentration, their ratios are also determined, namely N-acetylaspartate to creatine (NAA/Cr); choline to creatine (Cho/Cr) and choline to N-acetylaspartate (Cho/NAA) [10]. According to the statements of specialists dealing with cognitive disorders in various nosological units, the sensitivity, specificity, and positive predictive value for identifying cognitive deficits using MR spectroscopy are 89.8%, 88.2%, 95.3%, respectively [11]. There are many scientific works confirming the high importance of the above methods in the study of cognitive deviations associated with various diseases in both adults and children. However, despite this, quite a few questions remain that require more specific definitions and clarifications. This circumstance primarily concerns childhood and adolescence, when the problem of early diagnosis of cognitive deviations is especially acute and is of vital importance [12].Purpose of the study. Determining the role of biomarkers in the early diagnosis of cognitive deficits in children and adolescents with type 1 diabetes.
2. Material and Methods
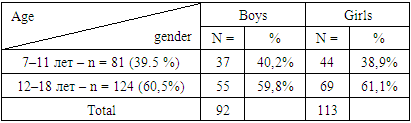
- A survey was conducted of 205 children aged 7 to 18 years suffering from diabetes mellitus (type 1 diabetes), 92 (45%) patients were boys, 113 (55%) patients were girls. Children from 7 to 11 years old (average age - 9.0±1.6 years) made up 81 patients (39.5%), patients from 12 to 18 years old, 124 patients were examined (average age - 14.7 ±1.8 years) – (60.5%) (Table 1).
|
3. Results and Discussion
- In a comparative assessment of HbA1c values taking into account the duration of the disease, we found that in the first 1.5-2 years from the onset of diabetes mellitus, glycated hemoglobin levels were significantly lower and averaged 8.5%. In the group with an experience of 3 to 6 years, more precisely, by the middle of the third year of diabetes duration, carbohydrate metabolism indicators worsened and their average values were significantly higher than in children with a duration of up to 3 years - 9.4% (p < 0.001). In patients with longer durations of diabetes, the trend towards worsening HbA1c levels did not decrease and on average was significantly higher - 10.4% (p < 0.001) than in groups with shorter durations of the disease.During the analysis of individual tasks for the MoCA test, it was determined that patients with type 1 diabetes performed significantly worse on the tasks “clock” (p<0.001), “attention” (p<0.001), and “repetition of a phrase” (p<0.001) and “delayed reproduction” (p<0.001) in relation to the control group (Figure 1.).
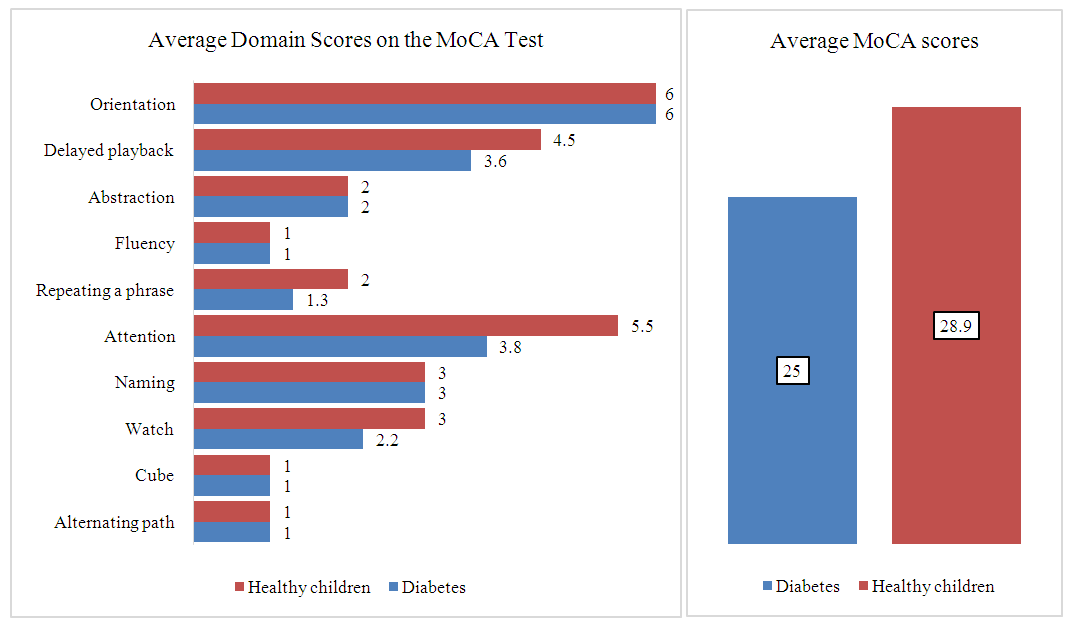 | Figure 1. Average score across MoCA domains depending on presence of diabetes |
|
|
4. Conclusions
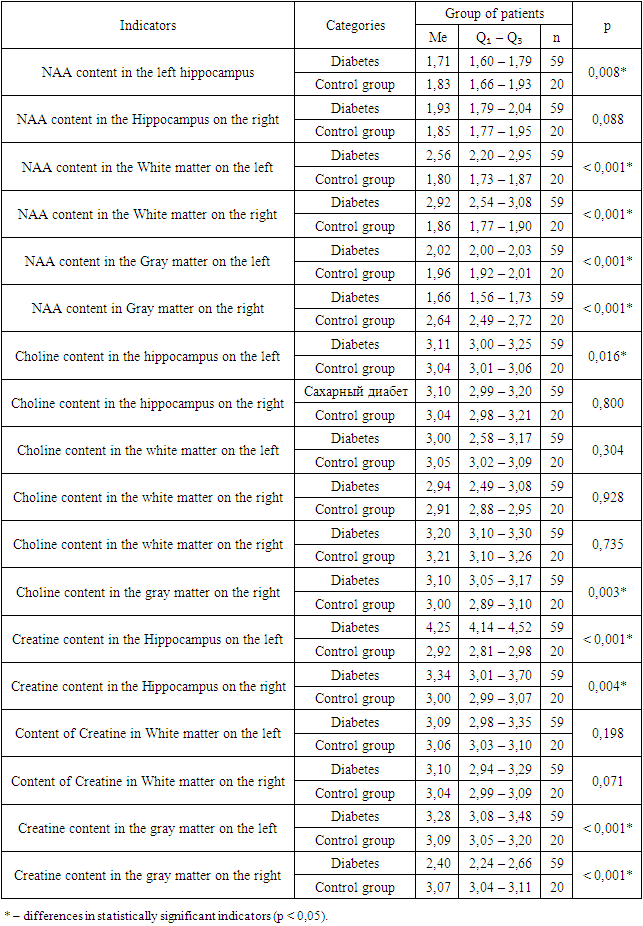
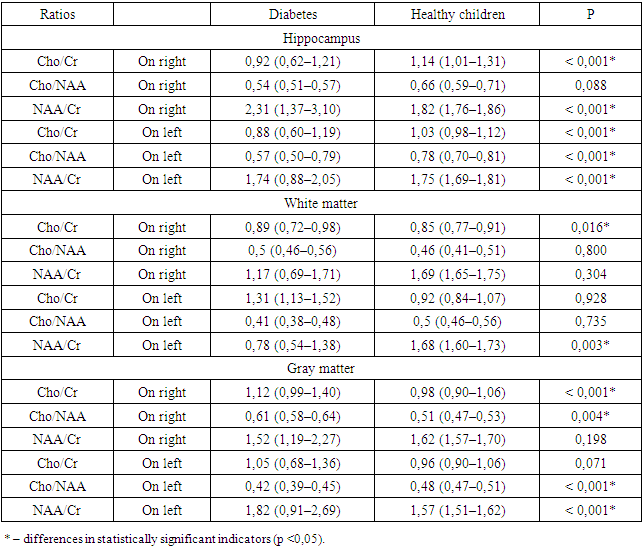
- Thus, a significant increase in the level of protein S-100 in the blood serum in children and adolescents with cerebral changes in type 1 diabetes and a noticeable correlation (p <0.001*) of this component with the MoCa test indicates an increased intensity of reactive gliosis with increasing the severity of brain disorders. In addition, an increase in the level of S-100 in the blood indicates a direct influence of reactive gliosis on the processes of pathogenesis of the development of cerebral disorders in type 1 diabetes in children and adolescents. An increase in the level of NSE in the blood serum in children and adolescents with type 1 diabetes with the presence of dysfunctions affecting the brain indicates activation of anaerobic glycolysis in the central nervous system and increased permeability of neuronal membranes during the formation of cognitive deficit, especially in the early stages of diabetes, which also has of particular importance in clinical and pathogenetic processes. When analyzing MR spectroscopy in children and adolescents with type 1 diabetes, the main changes in metabolites were determined in areas directly related to the processes of cognitive activity - this is the hippocampus area, gray and white matter (subcortical structures, thalamus) of the brain. Thus, a statistically significant decrease in NAA content in the left hippocampus was revealed (p < 0.001*); increased NAA levels in the white matter on the left and right (p < 0.001*); decreased NAA content in the gray matter on the right (p < 0.001*); increased choline level in the left hippocampus (p = 0.016*); increased choline in the gray matter on the right (p=0.003*); increase in creatine in the hippocampus on the left (p < 0.001*); increase in creatine in the hippocampus on the right (p=0.004*); an increase in creatine in the gray matter on the left (p<0.001*) and a decrease in creatine in the gray matter on the right (p<0.001*). The results demonstrated, as well as the observed heterogeneous changes in the Cho/Cr ratios; Cho/NAA; NAA/Cr in patients with cognitive deficits in type 1 diabetes, in addition, the presence of correlations between the studied components may indicate intracellular changes with energy deficiency, as well as due to energy imbalance, disorders of the integrity of neurons and optimal functioning of nervous tissue in these areas, this is important both in the pathogenetic and clinical processes of changes in cognitive activity in type 1 diabetes.Conducting a PMRS study made it possible to localize the areas of the brain responsible for the decline in cognitive functions in children and adolescents with type 1 diabetes, in addition, to obtain more accurate information about metabolic changes in these areas of the brain. In general, this method makes it possible to determine the dynamics of the condition of patients with type 1 diabetes mellitus, the presence of cerebral changes in them, even at a stage when there are no clinical manifestations, in addition to monitoring the possible progression of the disease, as well as the adequacy of the selected therapy for correcting abnormalities higher cortical activity. Referring to the results obtained, we can conclude that the use of P-MRS of the brain in the early diagnosis of manifestations of cognitive and emotional disorders in this pathology is justified, since it is possible to trace changes in brain tissue in the form of microstructural damage and visualize deviations in brain metabolism leading to cerebral dysfunctions even at the preclinical stage.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML