-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2024; 4(2): 343-346
doi:10.5923/j.ajmms.20241402.39
Received: Jan. 16, 2024; Accepted: Feb. 5, 2024; Published: Feb. 12, 2024

Myocardial Injury Markers in Pregnant Women with COVID-19 Associated Myocarditis
Anvar Alisherovich Tukhtabaev1, Ahmad Khoshimovich Karimov2
1PhD, Republican Specialized Scientific and Practical Medical Center for Mother and Child, Uzbekistan
2Doctor of Medical Sciences, Professor of the Department of Obstetrics and Gynecology in Family Medicine, Tashkent Medical Academy, Uzbekistan
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

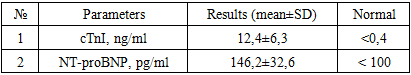
Purpose. The aim of this study was to evaluate the levels of laboratory markers, specifically troponin I and NT-proBNP, in pregnant women diagnosed with COVID-19-associated myocarditis, and to explore their correlation with the disease's severity. Methods. A cohort of 46 pregnant women diagnosed with COVID-19-associated myocarditis, based on comprehensive clinical, instrumental, and laboratory examinations, was identified from the selection results. Throughout the study, all pregnant women underwent a laboratory assessment of troponin I (cTnI) and N-terminal B-type natriuretic peptide (NT-proBNP) levels in their blood. Results. A 100% incidence of elevated cTnI levels was observed, with the average value in the group being 12.4±6.3 ng/ml, exceeding the reference value by 30 times. Similarly, NT-proBNP elevation was noted in all cases, with an average value in the sample of 146.2±32.6 pg/ml. Notably, in the majority of cases, NT-proBNP exceeded >128 pg/ml.Conclusion. NT-proBNP in the bloodstream serves not only as an indirect indicator of the presence of COVID-19-associated myocarditis but also as a criterion for assessing the severity of the condition. Furthermore, it acts as a predictive marker for the development of severe cardiovascular complications over time.
Keywords: COVID-19, Pregnancy, COVID-19 associated myocarditis, Laboratory markers, Troponin cTnI, NT-proBNP
Cite this paper: Anvar Alisherovich Tukhtabaev, Ahmad Khoshimovich Karimov, Myocardial Injury Markers in Pregnant Women with COVID-19 Associated Myocarditis, American Journal of Medicine and Medical Sciences, Vol. 4 No. 2, 2024, pp. 343-346. doi: 10.5923/j.ajmms.20241402.39.
1. Introduction
- Initially, it was believed that the SARS-CoV-2 virus primarily affects the respiratory system. However, it soon became clear that the infection also impacts other organs, including the cardiovascular system. The identification of cardiac involvement is crucial, as it signifies a significantly increased risk of in-hospital mortality (51% versus 4%) and adverse long-term clinical outcomes. Myocarditis is now recognized as a severe complication of COVID-19, prompting numerous research groups to focus on developing early diagnostic approaches and treatment strategies [1,2,3,4].Myocarditis is an inflammatory condition of the myocardium caused by various infectious and non-infectious agents. Viral etiology is still considered predominant in the United States, with documented cases linked to enteroviruses, including coxsackieviruses, parvovirus B-19, H1N1, and beta-coronaviruses such as MERS [5,6].The exact pathophysiology of SARS-CoV-2-associated myocarditis remains unclear. Depending on factors related to virus-host interactions and the phase of infection (acute, subacute, or chronic), proposed mechanisms may include (1) immune-mediated, (2) autoimmune, and (3) directly virus-induced pathways [6,10]. Immune-mediated myocarditis involves sudden or acquired immune reactions contributing to myocardial damage and extended cardiomyopathy consequences. Despite this, major clinical trials of immunosuppressive therapy using prednisolone and azathioprine have not shown significant clinical efficacy. However, there is some evidence suggesting that alternative immunomodulation strategies may be effective. Autoimmune myocarditis may develop in response to the release of genetically hidden antigens from cardiomyocytes, typically shielded from the immune system due to virus-induced damage [7,8].There is also supporting evidence [9,10] for the hypothesis that molecular mimicry, involving epitopes shared by viral capsid proteins, cardiac myosin, and other unspecified proteins on the surface of cardiomyocytes, stimulates autoimmune reactions. When viruses evade the immune system, they replicate and produce viral proteins that directly damage the myocardium, contributing to cellular apoptosis and necrosis. SARS-CoV-2 likely induces myocarditis in humans through one of these mechanisms, similar to other viral agents [11,12]. As discussed, SARS-CoV-2 can infect cardiomyocytes using their surface ACE2 receptors, causing direct cellular damage.Currently, there is accumulating information on extrapulmonary multiorgan manifestations of the novel coronavirus infection. It is evident that patients with pre-existing comorbidities are at risk for an unfavorable disease prognosis [9,10,11]. Myocardial injury emerging in the context of COVID-19 is of special concern to researchers, as it correlates with increased mortality [6,8]. Several pathogenetic mechanisms are presumed to be responsible for COVID-19-associated myocarditis development. The most likely is an immune-mediated mechanism triggered by a "cytokine storm." However, discussions continue on the direct cytopathic effect of the virus on cardiomyocytes. Endothelial dysfunction, disruption of the renin-angiotensin-aldosterone system (RAAS), and negative drug effects of medications used to treat SARS-CoV-2 infection also contribute to the overall understanding.Research Objective. To assess the levels of laboratory markers (troponin I and NT-proBNP) in pregnant women with COVID-19-associated myocarditis and their correlation with the severity of the disease.
2. Materials and Methods
- The study was conducted at the Republican Specialized Scientific-Practical Medical Center of Obstetrics and Gynecology from June 2020 to May 2022 in a prospective manner. The diagnosis of COVID-19-associated myocarditis in pregnant women was established based on the results of comprehensive clinical, instrumental, and laboratory examinations.Diagnostic Criteria included:Confirmed association of myocarditis with COVID-19 (confirmed by serological tests - PCR, ELISA (IgM, IgG)).Presence of characteristic clinical symptoms of myocarditis in pregnant women, such as heart failure syndrome.Typical changes on echocardiography (EchoKG) and cardiac MRI (presence of "Lake Louise Criteria (LLC)" - myocardial edema on T2 in T2 mapping of T2W images and non-ischemic myocardial damage on T1).Exclusion Criteria:Chronic cardiovascular diseases, including heart defects, rheumatism, and systemic connective tissue disorders.Following the selection process, a group of 46 pregnant women was identified, with an average age of 29.6±5.43 years and an average gestational age of 32.4±3.5 weeks.Laboratory AssessmentAll pregnant women underwent laboratory assessment of troponin I (cTnI) and N-terminal pro-B-type natriuretic peptide (NT-proBNP) levels in the blood during the study.Characteristics of NT-proBNPNT-proBNP is a precursor of brain natriuretic peptide. In the heart, small amounts of proBNP protein are produced, which are cleaved to form the active hormone - brain natriuretic peptide (BNP) - and an inactive fragment - N-terminal pro-brain natriuretic peptide (NT-proBNP). The hormone's function is to regulate the volume of circulating blood throughout the body and the force the heart must exert to pump blood. NT-proBNPis mainly produced in the left ventricle of the heart, which is the strongest "chamber" that performs the most work in pumping blood. Elevated concentrations of BNP and NT-proBNP in the blood result from increased workload on the left ventricle, often occurring in conditions like heart failure [10,13,14].Statistical AnalysisThe obtained results were subjected to statistical processing using Microsoft Office Excel-2019, including built-in functions for statistical analysis. Variational statistical methods were employed to calculate the mean (M), standard deviation (m), relative values (frequency, %), and statistical significance of the measurements. Student's t-test was used for comparing mean values, with calculation of the probability of error (p) during the normality test (based on kurtosis) and equality of population variances (F - Fisher criterion). Statistical significance was considered at a level of p<0.05.
3. Results
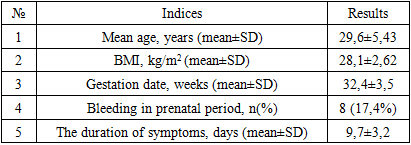
- The predominant share of somatic pathologies during the first half of gestation comprised varying degrees of anemia and acute respiratory infections (ARIs), identified in almost half of the women in the overall group (52.1%). Following them in frequency were respiratory diseases (15.2%), tonsillitis (10.8%), and arterial hypertension (17.3%). Therefore, these pathologies were considered contributing factors to development.General clinical indicators are presented in table 1. The average Body Mass Index (BMI) for pregnant women was 28.1±2.62 kg/m2, influenced by a relatively low percentage of women with obesity, which significantly impacts the development of heart failure symptoms. Bleeding episodes during the prenatal period were observed in 17.4% of women in the sample, attributed to various pathological conditions. The average duration of heart failure symptoms in pregnant women in the sample was 9.7±3.2 days. Upon admission, 31 (67.3%) patients reported shortness of breath, 8 (17.3%) reported palpitations, 10 (21.7%) reported decreased fetal movement, and 9 (19.6%) reported pronounced fatigue and weakness.
|
|
|
4. Discussion
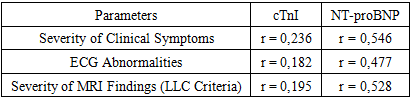
- Numerous studies demonstrate the detrimental impact of COVID-19 on the cardiovascular system. In women, COVID-19 is associated with cardiovascular diseases such as myocarditis, acute myocardial infarction, cardiomyopathy, arrhythmias, and venous thromboembolic complications [2,8,10,11]. The diagnosis of "myocarditis" poses significant challenges due to the peculiarities of the clinical picture and the nonspecific nature of the symptoms. The term "myocardial injury," based on the literature's definition of increased levels of cardiac troponin, is essentially a more comprehensive concept than "myocarditis," as it can also include conditions caused by myocardial ischemia and cardiomyopathies. Additional diagnostic procedures such as ECG, echocardiography (EchoKG), MRI, and ultimately endomyocardial biopsy are necessary to confirm the diagnosis of "myocarditis." However, performing these procedures is not always feasible in COVID-19 hospitals. Drug therapy for patients with inflammatory myocardial damage associated with a new coronavirus infection has not been developed. According to several meta-analyses, patients receive treatment according to COVID-19 management protocols, and the impact of most drugs on the course of myocarditis is unknown. Many drugs used to treat patients with a new coronavirus infection have potentially negative effects on the myocardium and can themselves provoke myocardial injury. Myocardial involvement is the most commonly reported cardiovascular complication in patients with COVID-19 and is associated with high mortality. Myocardial injury with elevated cardiac marker levels develops in 7-17% of patients hospitalized with COVID-19 and in 22-31% of more severe cases admitted to the intensive care unit [6,7]. In a cohort study of 191 hospitalized COVID-19 patients, 33 (17%) developed myocardial injury, and 32 (97%) of them died. Similar high mortality can be explained by the fact that 63% of COVID-19 patients had a severe or critical illness [9]. In another study [14], 20% of COVID-19 patients developed myocardial injury and were 5 times more likely to require mechanical ventilation and 11 times more likely to die than patients without cardiac complications. There are several mechanisms of myocardial injury, with myocarditis or systemic inflammation being the most common [1,3]. It should be noted that studies on pregnant women with COVID-19, examining the impact of COVID-19 on the cardiovascular system, are limited. Currently, there is only one study [9] demonstrating the influence of COVID-19 on the cardiovascular system during pregnancy. In this case series, two pregnant women with COVID-19 developed cardiac dysfunction with moderate reduction in left ventricular ejection fraction and its hypokinesia. Both patients had no complaints before, had no cardiovascular risk factors such as race and ethnicity or obesity, and one of them was in the late stages of pregnancy. Both patients gave birth by cesarean section and were in isolation rooms with negative pressure. The results are still unknown as they were still hospitalized in the intensive care unit at the time of writing, on the path to recovery. Similarly, in our study, myocardial injury with reduced left ventricular ejection fraction developed in 46 women, ranging from 22% to 45% with an average of 37.67%. In all cases, high levels of troponin and natriuretic peptide were present in the blood, with or without ECG changes. The increase in troponin levels in COVID-19 was directly proportional to the unfavorable course and high mortality. The presence of increased troponin concentrations is associated with a severe course of the disease and adverse outcomes in patients with COVID-19. Patients with mild forms of the disease rarely had elevated troponin levels. The National Health Commission of China reported that 12% of COVID-19 patients had elevated troponin levels. Moreover, they noted that 46% of COVID-19 patients who died had elevated troponin levels, while survivors had a rate of 1%. These results confirm the association of increased troponin levels with increased mortality [4,14]. Elevated levels of NT-proBNP in patients with COVID-19 are a marker of myocardial injury and are associated with in-hospital mortality. Guo's study and colleagues showed that increased levels of troponin and NT-proBNP were closely related to each other. In addition, Shi's study and colleagues [14] showed increased levels of NT-proBNP in COVID-19 patients with heart involvement compared to patients without heart involvement. These patients also had significantly higher mortality, which was 51.2%. Therefore, regular measurement of these biomarkers upon admission would play a crucial role in reducing mortality in patients at high risk.
5. Conclusions
- Thus, the assessment of laboratory markers in pregnant women with COVID-19-associated myocarditis showed that NT-proBNP in the blood is not only an indirect indicator of the presence of this pathology but also a criterion for the severity of the condition. It serves as a predictor for the development of severe complications from the cardiovascular system dynamically.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML