-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2024; 4(2): 340-342
doi:10.5923/j.ajmms.20241402.38
Received: Jan. 12, 2024; Accepted: Feb. 3, 2024; Published: Feb. 12, 2024

Characteristics of Hypoxic Injuries to the Nervous System in Newborns with Apnea
Dilmurodova Klara1, Ikromova Zarina2, Ziyadullaeva Hulkar2, Xudayberdieva Shakhnoza2
1DSc., Professor, Samarkand State Medical University, Samarkand, Uzbekistan
2Ph.D., Samarkand State Medical University, Samarkand, Uzbekistan
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The authors have conducted ultrasonic studies of the central nervous system in newborns with apnea. For the early detection of changes in the brain structures, neurosonographic examination of the central nervous system is recommended for all newborns at risk.
Keywords: Apnea, Hypoxia, Asphyxia, Depth of anterior horns of lateral ventricles, Intraventricular hemorrhage, Brain edema
Cite this paper: Dilmurodova Klara, Ikromova Zarina, Ziyadullaeva Hulkar, Xudayberdieva Shakhnoza, Characteristics of Hypoxic Injuries to the Nervous System in Newborns with Apnea, American Journal of Medicine and Medical Sciences, Vol. 4 No. 2, 2024, pp. 340-342. doi: 10.5923/j.ajmms.20241402.38.
1. Introduction
- The issue of caring for premature newborns is one of the pressing challenges in contemporary neonatology and the most urgent in perinatology [2]. The progressive development and improvement of perinatal technologies in recent times have made it possible to save the lives of extremely premature infants. A complication of intensive therapy for surviving preterm infants is the development of lung pathology - bronchopulmonary dysplasia, with a frequency of 44% in the group of children with extremely low body weight [1]. Respiratory diseases occupy a leading place in the structure of morbidity and mortality among newborns. Among newborns, respiratory disorders rank second in terms of morbidity. More than half of the children with respiratory disorders suffer from Respiratory Distress Syndrome (RDS). The frequency of RDS depends on the rate of preterm births and decreases with increasing gestational age. RDS is much more common in infants born before 28 weeks of gestation (incidence in this group is up to 80%) and remains a serious problem in 25% of children born before 34 weeks. The consequences of the respiratory distress syndrome in preterm infants are the cause of 30-50% of neonatal mortality [3].The development of respiratory disorders is often associated with perinatal hypoxia. The frequency of perinatal hypoxia shows no tendency to decrease. 60-80% of all central nervous system (CNS) disorders in children are related to perinatal hypoxia [2]. According to the literature, about half of the children first recognized as disabled are patients with cerebral palsy (CP), most of whom have a perinatal hypoxic-ischemic origin [8].In the structure of childhood disability, CNS disorders account for 50%, of which 40% are children with disabilities due to perinatal CNS lesions [8]. More than half of all cases of early childhood CNS function disorders are not due to acute hypoxia during childbirth, but to prolonged, chronic hypoxia of the fetus and newborn [5,7]. Perinatal CNS lesions are characterized by high prevalence, severe disability, and high newborn mortality (40-50%) due to perinatal brain lesions. Perinatal stroke occurs at a rate of 1:4000 newborns. Perinatal injuries of the nervous system lead to disability in 15-30% of full-term newborns and 40-60% of preterm infants [4]. Literature data indicate that 73% of all lesions are caused by various forms of hypoxia, 13% by CNS developmental anomalies, 7% by mechanical injuries during childbirth, and 7% are associated with infectious diseases of the brain membranes [6].Research Objective: The aim of this study was to investigate the clinical manifestations and neurosonographic features in newborns with apnea.
2. Materials and Methods
- The study was conducted at the Samarkand Regional Perinatal Center in the Department of Pathology and Resuscitation of Newborns.The criteria for including children in the study groups were: birth weight less than 2500 grams and gestational age less than 37 weeks.The first group comprised 35 conditionally premature healthy newborns from healthy mothers aged 20 to 35 years, without a complicated obstetric history, with physiological pregnancy and childbirth.The second group consisted of 35 premature infants born to mothers with a complicated obstetric history. This group was characterized by low scores on the Apgar scale (1-3 points in 60% of children), a 100% history of complicated obstetrical-gynecological issues, and more pronounced signs of immaturity (such as lanugo, underdeveloped nails, poor development of subcutaneous fat, etc.). The condition of the children at birth was severe (66%) or extremely severe (34%), with suppression of physiological reflexes and requiring prolonged respiratory support. These children were nursed in the department of neonatal resuscitation and intensive care. Neurological symptoms in these children included lack of response to examination and pain stimuli, adynamia, areflexia, atonia, sluggish or absent pupillary reaction to light, and occasionally local ocular symptoms. The skin was cyanotic, pale with a "marbled tone" (34%) (indicative of microcirculation disorders).All examined had independent shallow breathing with intercostal retraction, episodes of apnea lasting more than 20 seconds, and bradycardia in 100% of the second group's preterm infants. Heart sounds were muffled (86%), and abdominal palpation showed moderate liver enlargement of 3 cm in 57% of the children.The diagnosis of perinatal encephalopathies, depending on the nervous system lesions, was established according to the Sarnat and Sarnat classification of perinatal nervous system injuries in newborns.Ultrasonographic examination of the brain structure in B-mode (neurosonography) was performed using the GE Logic F 8 device (USA) with multi-frequency convex transducers of 5.5 MHz.Statistical processing of the data was carried out using the SPSS software (version 29, IDV Co., Armonk, NY, USA).
3. Results and Discussion
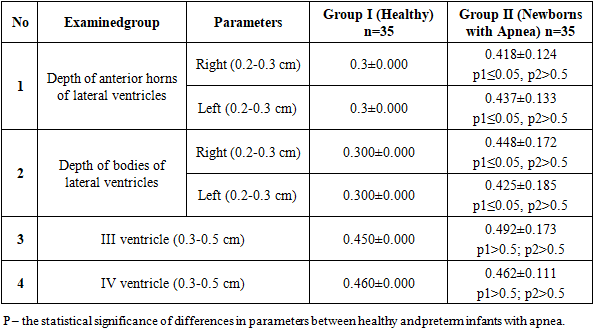
- In the study of neurosonographic parameters, such as the depth of the anterior horns (right and left) and the depth of the bodies of the lateral ventricles (right and left), differences were observed in healthy newborns and those with apnea, which showed statistically significant variations. However, the measurements of the III and IV ventricles in healthy and apnea-affected children did not show a statistically significant difference. For instance, the depth of the anterior horns of the lateral ventricles on the right in healthy newborns was 0.3 cm, compared to 0.418±0.124 cm in newborns with apnea (p≤0.05). On the left, the depth of the anterior horns of the lateral ventricles was 0.3 cm in healthy newborns and 0.437±0.133 cm in those with apnea, with a statistically significant difference (p≤0.05).The depth of the bodies of the lateral ventricles on the right in healthy newborns was 0.3 cm, while in those with apnea, it was 0.448±0.172 cm (p≤0.05). On the left, this depth was 0.3 cm in healthy newborns and 0.425±0.185 cm in those with apnea (p≤0.05). The parameters of the III and IV ventricles in healthy newborns and children with apnea did not demonstrate a statistically significant difference (Table 1).
|
4. Conclusions
- Thus, preterm infants who have experienced apnea are at risk for perinatal injury to the nervous system. For early detection and timely staged treatment of preterm infants with hypoxic injuries to the nervous system, neurosonographic examination of the brain structures is recommended for all preterm infants at risk.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML