-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2024; 4(2): 310-315
doi:10.5923/j.ajmms.20241402.32
Received: Jan. 13, 2024; Accepted: Feb. 2, 2024; Published: Feb. 8, 2024

Clinical and Laboratory Characterization of Sars-Cov-2 Infection
Niyazov G. E., Oblokulov A. R.
Bukhara State Medical Institute, Bukhara, Uzbekistan
Correspondence to: Oblokulov A. R., Bukhara State Medical Institute, Bukhara, Uzbekistan.
| Email: |  |
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

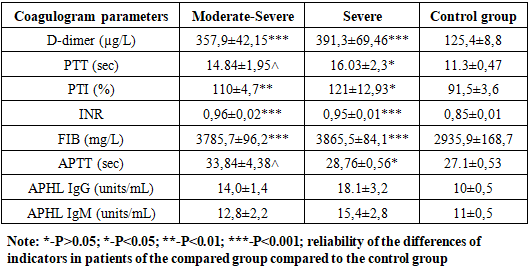
The significance of D-dimer coagulation (DD), prothrombin time (PT), activated partial thromboplastin time (APTT), thrombin time (TT) and fibrinogen (Fg) and PCT in predicting the severity and prognosis of COVID-19 was studied. Violation of the function of blood coagulation occurred in almost all, more often in severe patients. Indicators of hemostatic homeostasis, such as D-dimer, prothrombin time, fibrinogen and procalcitonin, can be used as predictors of disease severity in patients.
Keywords: COVID-19, Prothrombin time, D-dimer, Antiphospholipid antibodies, Procalcitonin
Cite this paper: Niyazov G. E., Oblokulov A. R., Clinical and Laboratory Characterization of Sars-Cov-2 Infection, American Journal of Medicine and Medical Sciences, Vol. 4 No. 2, 2024, pp. 310-315. doi: 10.5923/j.ajmms.20241402.32.
Article Outline
1. Introduction
- A new coronavirus infection (СoronaVIrus Disease 2019 – COVID-19) is an infection caused by SARS-CoV-2 (Severe Acute Respiratory Syndrome-related CoronaVirus-2) coronavirus. According to the latest statistics from the World Health Organization (WHO), the disease has already covered all continents, with more than 233,739,828 diagnosed cases in 221 countries and almost 4,782,139 deaths as of September 28, 2021 [1]. The spectrum of clinical manifestations that occur with COVID-19 includes fever, myalgia, cough and shortness of breath, and less often headache, diarrhea, nausea and vomiting [2].Coronavirus infection (COVID-19) is characterized by activation of the hemostasis system, which in the most severe cases may lead to consumption coagulopathy. At present, it remains unclear whether COVID-19 is the direct cause of these disorders or whether they occur as the infectious process progresses [3,4].Most patients with COVID-19 develop symptoms of a respiratory infection, in some of them they worsen to a more severe systemic illness characterized by persistent fever, acute lung injury with acute respiratory distress syndrome, multiple organ failure, shock, and high mortality [5,6]. Careful monitoring of patients with COVID-19 showed that many of them had abnormalities in laboratory tests of the blood coagulation system, resembling other systemic coagulopathies, such as disseminated intravascular coagulation (DIC) and thrombotic microangiopathies [7,8].In addition, it turned out that COVID-19-associated coagulopathy also has features that distinguish it from DIC and TMA [9].Coagulation disorders have been reported in COVID-19 patients in several descriptive studies [10,11,12]. Elevated levels of D-dimer and fibrin degradation products (FDP), shortened or increased prothrombin time (PT), abnormal platelet count, occurrence of thrombosis or bleeding, and complications of disseminated intravascular coagulation have been observed in patients with COVID-19 at various clinical stages [13,14,15]. These data show that clotting disorders play an important role in the clinical process of COVID-19. Coagulation failure in end-stage COVID-19 or after invasive treatment is common and reasonable, but with limited predictive value. On the early hospitalization, more attention should be paid to coagulation function, which can help clinicians identify high-risk patients and guide clinical strategy.In COVID-19, initial reports of the benefit of PCT came from hospitalized patients in whom PCT was found to correlate with disease severity, longer intensive care unit (ICU) stay, and hospital mortality, as well as a number of other biochemical markers [16,17,18].Procalcitonin in coronavirus infection with damage to the respiratory sections of the lungs is within the reference values [19]. An increase in PCT indicates the addition of a bacterial infection and correlates with the severity of the course, the prevalence of inflammatory infiltration, and the prognosis for bacterial complications.Accumulating evidence shows that more than 80% of COVID-19 patients are receiving antibiotic treatment, as it is difficult to identify patients with COVID-19 without an underlying bacterial infection who can safely stop taking antibiotics. However, recent clinical evidence suggests that procalcitonin may help evaluate these patients and reduce the unnecessary use of antibiotics [20,21].
2. Materials and Methods
- This study was a single center retrospective cohort study. We included all patients with confirmed SARS-CoV-2 infection admitted to the Infectious Diseases Hospital in Bukhara. Clinical data were obtained from electronic health records including demographics, exposure history, signs and symptoms, as well as laboratory data at admission. Upon admission to the inpatient emergency department, all patients were assessed using the NEWS scale. The mean score was 5.6±1.6. This made it possible to quickly triage patients and refer the most severe patients to the intensive care unit. All patients with COVID-19 included in this study were diagnosed according to the guidelines for the diagnosis and treatment of pneumonia caused by novel coronavirus infection. All patients had laboratory-confirmed SARS-CoV-2 infection (real-time RT-PCR, specific for SARS-CoV-2, was positive).Clinical and laboratory data of 120 patients were studied. Patients were divided into severe patients (n=60) and patients with moderate forms (n=60). Of these, 12 (20.0%) patients were admitted to the intensive care unit.The median age was 53 years, and of the 120 patients, 96 (80%) were men. The median time from symptom onset to hospitalization was 2–3 days, and the median time to diagnosis of severe illness was 3–4 days. Routine blood tests: white blood cell count (WBC), lymphocyte count (LYM), mononuclear cell count (MONO), neutrophil count (NEU) were performed on blood samples. Blood biochemistry parameters: aspartate aminotransferase (AST), alanine aminotransferase (ALT), glucose (GLU), urea, creatinine and C-reactive protein (CRP) were measured using an automatic biochemical analyzer MINDRAY BC-30 (China). Coagulation functions (prothrombin time (PTT), fibrinogen (FIB), activated partial thromboplastin time (APTT) were determined using the analyzer MINDRAY BA - 88A (China). The concentration of D-dimer was determined using the ELISA method using reagent kits for enzyme immunoassay for determining the concentration of D-dimer in blood plasma D-dimer - ELISA-BEST. The concentration of antibodies to phospholipids IgM/IgG were determined using the ELISA method.The concentration of PCT was determined using the ELISA method using reagent kits for enzyme immunoassay for determining the concentration of PCT in the blood plasma of PCT - ELISA-BEST. Moderate and severe patients used data from their first laboratory test on day 2 on admission, day 3 and 5 of treatment.The concentration equal to 0.05 ng/ml was taken as the upper limit of the norm. The obtained data were compared with the stock ones in the recommendations for the clinical interpretation of the results of determining the level of PCT in the blood serum: 0.1–0.25 ng/ml - the probability of a bacterial infection is very low; 0.25-0.5 ng / ml - a local bacterial infection is possible; 0.5-2.0 ng / ml - a high probability of a bacterial infection, a systemic bacterial infection is possible; 2.0-10.0 ng / ml - high probability of systemic bacterial infection, severe sepsis is possible; >10.0 ng/ml – high probability of severe sepsis [22].
3. Results of the Study and Discussion
- When analyzing the patients in the study groups by gender, the following results were obtained: there were 23 (46.9%) men with the moderate form of COVID-19 and 26 (53.1%) women. There were 33 (46.5%) males and 38 (53.5%) females with severe form of COVID-19.The results of the study showed that patients with moderate COVID-19 had cough, 49% - had sputum, 51% - had dyspnea, 59.2% - had sore throat, 87.8% - had anorexia, 6.1% - diarrhea, 10.2% - nausea, 6.1% - vomiting, 6.75% - abdominal pain, 83.7% - headache, 81.6% - fatigue, 26.5% - dizziness, loss of smell and taste in 28.6%, myalgia in 16.3%, confusion in 12.2%, conjunctivitis in 22.4%, lacrimation in 8.2%, and various skin rashes in 18.4%. And with severe COVID-19 there was cough, sputum discharge in 57.7%, dyspnea in 57.7%, sore throat in 64.8%, anorexia in 18.3%, diarrhea in 18.3%, nausea in 18.3%, vomiting in 12.7%, abdominal pain in 8.3%, headache in 90.1%, fatigue in 90.1%, dizziness in 33.8%, loss of smell and taste in 35.2%, myalgia in 12.7%, confusion in 12.7%, conjunctivitis in 34.7%, lacrimation in 11.3%, various skin diseases in 22.5%, various rashes.Elevation of body temperature is one of the main symptoms, 63.3% of patients with moderate form had body temperature ≤37.9°C, 36.7% had ≥38°C, 33.8% of patients with severe form had ≤37.9°C and 66 was seen as ≥38°C in 2%.Leukocytes averaged 7.13±0.5x109/L in patients with moderate form of the disease, control group patients averaged 5.01±0.14x109/L, lymphocytes averaged 1,34±0.11%, in the control group - on average 1.31±0.12%, monocytes - on average 0.37±0.03%, and in the control group - on average 0.6±0.04%, erythrocytes - on average 4.64±0.09x1012/l, and in control group patients 4, 65±0,13x1012/l, hemoglobin 139,7±3,3 g/l, in control group patients 137,35±2,56 g/l, hematocrit index 0,39±0,009%, in control group 0, 44±0,02 g/l, platelets 194±12,4x109/l, 237,75±16,4x109/l and in control group patients 16,4x109/l, COE 22,2±1,8 mm/s, in control group patients 5,35±0,57 mm/s.ALT and AST, which are considered to be important indicators of blood biochemical analysis, increased about 2.5 times in ALT study (52,96±5,5 U/l) in patients with the moderate form of the disease in comparison with the results recorded in patients of the control group (22±2,46 U/l). In patients with severe form of the disease (60,7±6,25 U/L) this index is higher than in patients with moderately severe form of the disease and almost 3 times higher than in patients of the control group (Fig. 1).
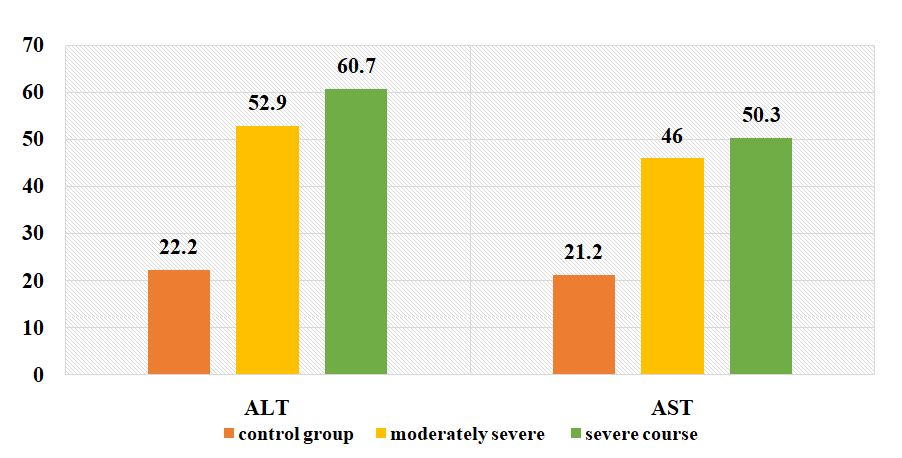 | Figure 1. Changes in the amount of ALT and AST depending on the severity of the disease in the examined patients (U/L) |
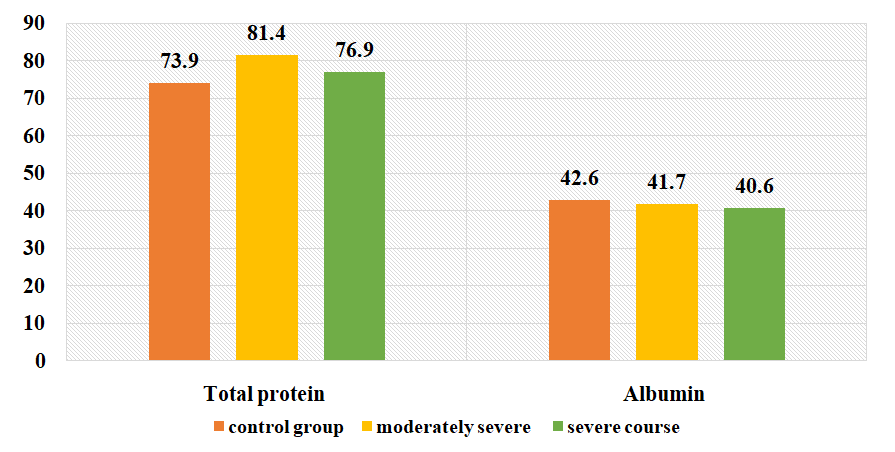 | Figure 2. Changes in the amount of total protein and albumin (g/l) in the examined patients depending on the severity of the disease |
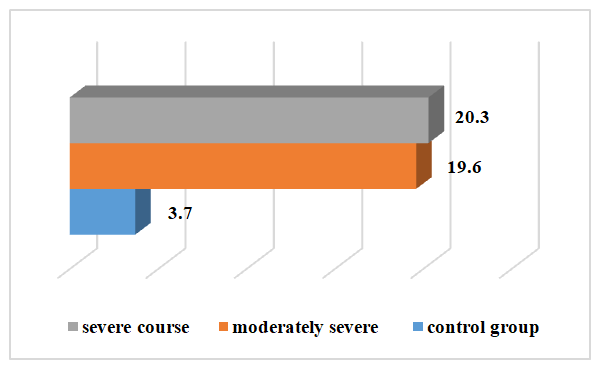 | Figure 3. Changes in the amount of CRP in the examined patients depending on the severity of the disease before treatment |
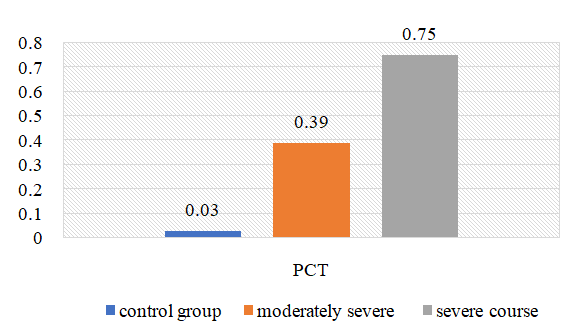 | Figure 4. Change in the amount of PCT in the examined patients depending on the severity of the disease (ng/L) |
|
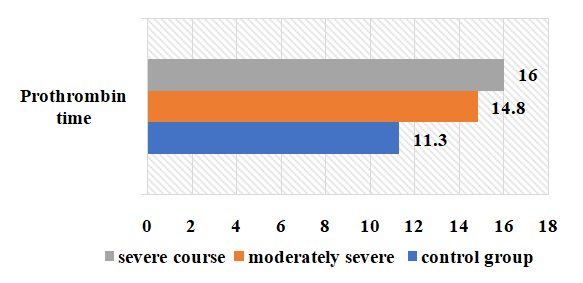 | Figure 5. Change of prothrombin time in the examined patients depending on the severity of the disease (sec) |
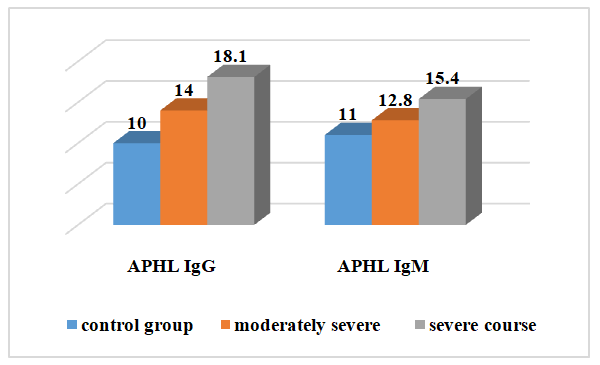 | Figure 6. Changes in the amount of APHL IgM and IgG in the examined patients depending on the severity of the disease before treatment |
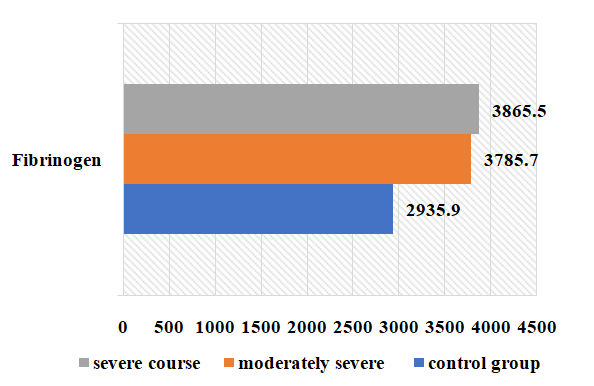 | Figure 7. Variation of fibrinogen in the examined patients depending on the severity of the disease (g/l) |
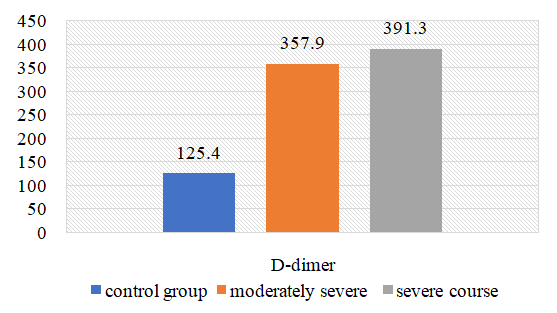 | Figure 8. Changes in the amount of D-dimer in the examined subjects |
4. Conclusions
- 1. When comparing the clinical course of coronavirus infection in patients with severe form of the disease, cough is 1.8 times more frequent than in the moderate form of the disease, sputum separation is 1.7 times more frequent, dyspnea and sore throat are 1.6 times more frequent, anorexia is 1.5 times more frequent, headache and fatigue are 1.6 times more frequent. In biochemical blood analysis the content of total protein was 1.1 times higher (81.5 g/l), ALT - 60.7±6.25 units/l, AST - 50.3±5. It was found that ALT and AST increased 1.2 and 1.1 times, respectively, in patients with a moderate form of coronavirus infection reaches 0.2 units/l. This indicates liver damage in patients with a moderate form of coronavirus infection. It was found that the amount of procalcitonin in patients with severe form of the disease reached 0.75±0.03 ng/mL, which is 2 times higher than in patients with moderate form of the disease, and bacterial infection was added to the disease. disease in these patients.2. It was found that partially activated thromboplastin time was increased 1.2 times in patients with a moderate form of coronavirus infection compared to patients with a severe form, and prothrombin index, on the contrary, was increased 1.1 times in patients with a severe form. of the disease compared to patients with a moderate form of the disease, and the occurrence of coagulopathy at a severe level of this disease indicates a high frequency. Testing of APHL IgG and APHL IgM in patients allows predicting the occurrence of blood coagulation disorders in patients with coronavirus infection.ConclusionsThus, such indicators of hemostatic homeostasis as platelet levels, D-dimer, fibrinogen and antiphospholipid antibodies are predictors of COVID - 19 associated coagulopathy and indicate the severity of the disease in patients. Further research is required to better understand the pathogenesis of COVID-19 associated coagulopathy. Procalcitonin is a biomarker for risk assessment of bacterial infection and disease progression.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML