-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2024; 4(2): 259-262
doi:10.5923/j.ajmms.20241402.20
Received: Jan. 11, 2024; Accepted: Feb. 6, 2024; Published: Feb. 8, 2024

Prognostic Effectiveness of Carrying Genotypic Variants of Gene Polymorphisms VEGFA (Rs2010963) and HIF1A (Rs11549465) in the Development of Dopamine Agonist Resistant Prolactinomas
Z. Yu. Khalimova, M. S. Safarova
The Republican Specialized Scientific And Practical Medical Center Of Endocrinology Named After Academician Y.H. Turakulov, Tashkent, Uzbekistan
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

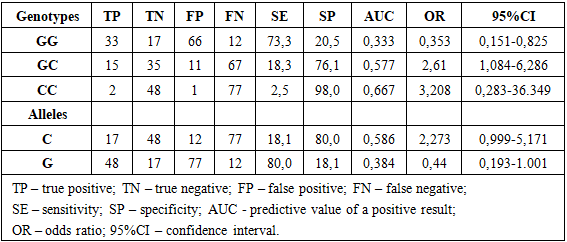
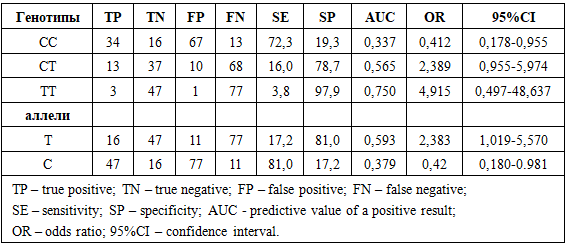
Research objective. To evaluate the prognostic effectiveness of carrying genotypic variants of VEGFA (rs2010963) and HIF1A (rs11549465) gene polymorphisms in the development of resistance of prolactinomas to dopamine agonist therapy. Methods. The study included cases of hormonally active prolactinomas (PRL) confirmed by clinical, laboratory, and instrumental investigations. Based on the selection results, a total sample of 128 PRL cases was formed. A prospective cross-sectional study included the randomization of patients with hormonally active PRL depending on PRL sensitivity to therapy. Gene polymorphisms VEGFA (rs2010963) and HIF1A (rs11549465) were examined using allele-specific polymerase chain reaction with SNP Express reagent kits in real-time mode. Results. The research results indicated that the GC genotype of the VEGFA gene polymorphism (rs2010963) exhibits moderate sensitivity (18.3%) and high specificity (76.1%), with the highest prognostic effectiveness. This is confirmed by a relatively high odds ratio with a confidence interval upper limit >1, highlighting its prognostic value. The CT genotype of the HIF1A gene polymorphism (rs11549465) demonstrates moderate sensitivity (16%) and high specificity (78.7%), with the highest prognostic effectiveness. Conclusion. Relatively high prognostic effectiveness has been established for carrying the GC genotype and C allele of the VEGFA gene polymorphism (rs2010963), as well as the CT genotype and T allele of the HIF1A gene polymorphism (rs11549465) in the development of prolactinoma resistance to dopamine agonist therapy.
Keywords: Prolactinoma, Dopamine agonist resistance, VEGFA (rs2010963) polymorphism, HIF1A (rs11549465) polymorphism
Cite this paper: Z. Yu. Khalimova, M. S. Safarova, Prognostic Effectiveness of Carrying Genotypic Variants of Gene Polymorphisms VEGFA (Rs2010963) and HIF1A (Rs11549465) in the Development of Dopamine Agonist Resistant Prolactinomas, American Journal of Medicine and Medical Sciences, Vol. 4 No. 2, 2024, pp. 259-262. doi: 10.5923/j.ajmms.20241402.20.
1. Introduction
- Genetic features inherent in hormonally active pituitary adenomas, including prolactinomas, have remained largely understudied [1,2]. In recent years, there has been a considerable amount of information regarding the genetic changes in prolactin-secreting pituitary adenomas. The search for genes associated with the formation of prolactinomas resistant to dopamine agonist therapy continues [3,4,5,6].However, research on the genetic characteristics of prolactin-secreting pituitary adenomas with resistance to drug therapy remains limited at present. There are research results dedicated to mutations associated with prolactinoma resistance to dopamine agonist treatment [7,8,9].Studying the molecular profile of prolactin-secreting pituitary adenomas is necessary to understand the key factors that form the basis of tumor development and its resistance to treatment. The application of molecular genetic methods in the early stages of treatment allows for determining a rational personalized management strategy for patients. In certain cases, the results of these studies enable the identification of indications for surgical treatment, prevention of negative changes in the topography and anatomy of the adenoma, and reduction of the risk of surgical intervention [5,10,11].In connection with the above, the aim of this study was to evaluate the prognostic effectiveness of carrying genotypic variants of gene polymorphisms VEGFA (rs2010963) and HIF1A (rs11549465) in the development of prolactinoma resistance to dopamine agonist therapy.
2. Materials and Methods
- The study was conducted from 2021 to 2024. The research took place at the Republican Specialized Scientific and Practical Medical Center of Endocrinology named after academician Y.X. Turakulov, Ministry of Health of the Republic of Uzbekistan. Clinical, imaging, and laboratory stages of the study were conductedwithin the framework of the research. The molecular-genetic stage of the study was carried out at the laboratory of the molecular-genetic department of the Specialized Scientific and Practical Medical Center of Hematology, Ministry of Health of the Republic of Uzbekistan.Inclusion criteria:Cases of hormonally active prolactinomas (PRL) confirmed by clinical, laboratory, and instrumental studies:Microprolactinomas (less than 10 mm);Macroprolactinomas (more than 10 mm);Giant PRL (more than 4 cm).They included PRL with aggressive behavior (APRL) (recurrent, resistant, rapid growth, and imaging characteristics of aggressive growth) and therapy-resistant PRL (TR-PRL) (resistance to therapy was defined as the absence of normalization of prolactin blood levels and/or no reduction in adenoma volume by 50% or more from the baseline on maximum tolerable doses of dopamine agonists, but not less than 15 mg/day bromocriptine or 3 mg/week cabergoline, for at least 6 months).Exclusion criteria:Oncological history;Concurrent genetic diseases;Hypothyroidism and other endocrine disorders;Functional hyperprolactinemia;Non-functioning adenoma, corticotropinoma, somatotropinoma, gonadotropinoma, thyrotropinoma;Cirrhosis of the liver, chronic kidney disease, chronic heart failure.As a result of the selection, a total sample of PRL cases was formed, consisting of 128 patients, with an average age of 44.2±8.7 years. The prospective cross-sectional study included the randomization of patients with hormonally active PRL depending on PRL sensitivity to therapy (sensitive to dopamine agonists and resistant to therapy).Polymorphisms of the VEGFA (RS2010963) and HIF1A (RS11549465) genes were investigated using the allele-specific polymerase chain reaction (PCR) method with the SNP-Express reagent kits in real-time mode ("Syntol," Russia) PCR-RV (RT-PCR).The prognostic effectiveness of each genetic indicator was calculated by determining sensitivity (SE), specificity (SP), and AUC (positive predictive value). The AUC of genes was determined as follows: if the AUC value <0.5, the marker was considered random; 0.5
3. Results
- Table 1 presents the results of assessing the prognostic effectiveness of carrying genotypic and allelic variants of the VEGFA gene polymorphism (RS2010963) in the context of the development of prolactin resistance to dopamine antagonist therapy. For this purpose, the following indicators characterizing prognostic effectiveness were calculated: SE – sensitivity; SP – specificity; AUC - positive predictive value; OR – odds ratio; 95% CI – confidence interval.
|
4. Discussion
- Some researchers have found that hypervascularization plays a key role in the invasiveness and spread of various tumor types, including invasive pituitary adenomas. Studies have also examined the role of vascular endothelial growth factor (VEGF) expression and its receptors VEGFR-2 in pituitary adenomas, revealing a significant correlation between adenoma volume and VEGFA expression levels. In conclusion, angiogenesis in tumorigenesis is a complex and dynamic process, and while the vascularity of pituitary adenomas, especially prolactinomas, remains unknown, for invasive or aggressive pituitary adenomas, angiogenesis is considered one of the important pathogenetic mechanisms [2,4,12,13].Vascular endothelial growth factor (VEGF) is a signaling protein that promotes the formation of new blood vessels. VEGF plays a role in restoring oxygen supply to cells and tissues in cases of hypoxia caused by impaired blood circulation. Overexpression of VEGF contributes to tumor development by providing enhanced oxygenation to support continuous tumor growth. Tumors capable of producing VEGF can sustain their growth by oxygenating the expanding tissue, and this process is known as angiogenesis [1,7,14].Hypoxia-inducible factor-1 alpha (HIF-1α) has the ability to regulate the level of vascular endothelial growth factor (VEGF) not only in pituitary adenomas but also in other tumors. More than a decade ago, an experiment reducing HIF-1α levels demonstrated its protective function by reducing apoptosis in the human pituitary adenoma cell line (HP75) under hypoxic conditions. Subsequent studies confirmed that VEGF activation occurs under the influence of HIF-1α. It is reported that HIF-1α activates VEGF under hypoxic conditions, confirming the interaction between HIF-1α and VEGF. Studies have also identified excessive expression of HIF-1α and VEGF-A in postoperative surgical materials from invasive pituitary adenomas compared to non-invasive samples, confirming the role of HIF-1α as the top regulator of VEGF expression. All these findings contribute to the complex HIF-1α-VEGF pathway, activating invasive processes in pituitary adenomas [2,3,4,15].In addition, von Hippel-Lindau tumor suppressor (pVHL) protein is known as a negative regulator of HIF-1α. Low expression of this protein with high VEGF expression is associated with an increased recurrence rate and more aggressive behavior of pituitary adenomas. All these facts complement each other and confirm the importance of pathways related to VEGF and HIF-1α in the invasiveness and aggressiveness of pituitary adenomas [1,3,4].
5. Conclusions
- A relatively high prognostic efficiency of carrying the GC genotype and C allele of the VEGFA gene polymorphism (rs2010963), as well as the CT genotype and T allele of the HIF1A gene polymorphism (rs11549465), in the development of prolactin resistance to dopamine agonist therapy has been established. This allows for the use of these genetic markers for early prediction of prolactin resistance development to treatment.
References
| [1] | Chanson P, Maiter D. The epidemiology, diagnosis and treatment of Prolactinomas: The old and the new. Best Pract Res Clin Endocrinol Metab. 2019; 33. |
| [2] | Glezer A, Bronstein MD. Prolactinomas. Endocrinol Metab Clin North Am. 2015; 44: 71-78. |
| [3] | Huynh PP, Ishii LE, Ishii M. Prolactinomas. JAMA. 2021; 325: 195. |
| [4] | Faltermeier CM, Magill ST, Blevins LSJr, Aghi MK. Molecular Biology of Pituitary Adenomas. NeurosurgClin N Am. 2019; 30(4): 391-400. |
| [5] | Barry S, Korbonits M. Update on the genetics of pituitary tumors.EndocrinolMetabClin North Am. 2020; 49: 433-452. |
| [6] | Auriemma RS, Pirchio R, Pivonello C, Garifalos F, Colao A, Pivonello R. Approach to the Patient With Prolactinoma. J ClinEndocrinolMetab. 2023 Aug 18; 108(9): 2400-2423. |
| [7] | Gao H, Wang F, Lan X. et al. Lower PRDM2 expression is associated with dopamine-agonist resistance and tumor recurrence in prolactinomas. BMC Cancer. 2015; 15(1): 272. |
| [8] | Inder WJ, Jang C. Treatment of Prolactinoma. Medicina (Kaunas). 2022; 58(8). |
| [9] | Lasolle H, Ilie MD, Raverot G. Aggressive prolactinomas: How to manage? Pituitary. 2020; 23: 70-77. |
| [10] | Maiter D. Management of dopamine agonist-resistant prolactinoma. Neuroendocrinology. 2019; 109: 42-50. |
| [11] | Olarescu NC, Perez-Rivas LG, Gatto F, Cuny T, Tichomirowa MA, Tamagno G, Gahete MD. EYRC (ENEA Young Researcher Committee): Aggressive and malignant prolactinomas. Neuroendocrinology. 2019; 109: 57-69. |
| [12] | Petersenn S, Fleseriu M, Casanueva FF, Giustina A. Diagnosis and management of prolactin-secreting pituitary adenomas: a Pituitary Society international Consensus Statement. Nat Rev Endocrinol. 2023; 19(12).: 722-740. |
| [13] | Raverot G, Vasiljevic A, Jouanneau E, Lasolle H. Confirmation of a new therapeutic option for aggressive or dopamine agonistresistant prolactin pituitary neuroendocrine tumors. Eur J Endocrinol. 2019; 181: 1-3. |
| [14] | Souteiro P, Belo S, Carvalho D. Dopamine agonists in prolactinomas: When to withdraw? Pituitary. 2020; 23: 38-44. |
| [15] | Chen C, Yin S, Zhang S, Wang M, Hu Y, Zhou P, Jiang S. Treatment of aggressive prolactinoma with temozolomide: A case report and review of literature up to date. Medicine (Baltimore). 2017; 96. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML