-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2024; 14(1): 60-63
doi:10.5923/j.ajmms.20241401.14
Received: Dec. 19, 2023; Accepted: Jan. 9, 2024; Published: Jan. 11, 2024

Studying the Importance of Vitamin D Levels in Patients with Chronic Kidney Disease During Left Ventricle Remodeling Processes
Daminov B. T., Akbarov I. B.
Tashkent Pediatric Medical Institute, Tashkent, Uzbekistan
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The results of the study of EchoCG parameters indicate a significant contribution of Vit.D deficiency in patients with CKD. in the progression of LV remodeling. The results of the analysis of LV size indicators revealed the peculiarity of structural and geometric changes in the left parts of the heart with Vit.D deficiency in patients with CKD. Patients with CKD with Vit.D deficiency are characterized by more pronounced manifestations of cardiac remodeling and with a predominance of eccentric LVH.
Keywords: Chronic kidney disease, Vitamin D, Glomerular filtration rate, Left ventricular remodeling
Cite this paper: Daminov B. T., Akbarov I. B., Studying the Importance of Vitamin D Levels in Patients with Chronic Kidney Disease During Left Ventricle Remodeling Processes, American Journal of Medicine and Medical Sciences, Vol. 14 No. 1, 2024, pp. 60-63. doi: 10.5923/j.ajmms.20241401.14.
Article Outline
1. Introduction
- According to epidemiological studies, a violation of the homeostasis of phosphorus, calcium and vitamin D in chronic kidney disease (CKD) is detected already at an early stage of renal failure. The majority of patients with stage IIIB CKD have hyperphosphatemia and a relative deficiency in the blood of the active metabolite of vitamin D3 — calcitriol [1-3].However, to date there is no standardized approach to determining the initiation of AMDD therapy. The previously formed opinion that it is more expedient to start treatment with vitamin D after the start of dialysis than before dialysis [1,2] has been revised in recent years. A number of studies have proven the benefits of early correction of PTH hyperproduction in CKD.In isolated studies, it was found that the use of AMBD at the predialysis stages of CKD improves the quality of life of patients [7], can reduce the incidence of cardiovascular [7-10] and renal complications [11,12], provided that it was possible not only to achieve, but also to maintain the target levels of PTH. Moreover, the results of observational studies indicate a strict association of predialysis treatment of AMBD with the delay in the onset of dialysis, lower mortality of patients on dialysis compared with patients not treated with vitamin D at the prehospital stage with secondary hyperparathyroidism (SHPT), as well as a significant reduction in the cost of the treatment program for patients with CKD in general [3,7]. Data have been obtained that AMBD gives pleiotropic effects, including reno-cardioprotective [3,7].The purpose of the study: to study the value of vitamin D levels in patients with stage 2-3 CKD, as well as the relationship between vitamin D levels and indicators of markers of bone mineral metabolism.
2. Materials and Methods of Research
- 105 patients with chronic kidney disease (CKD) stages C2 and C3, aged 35 to 69 years, of inflammatory and vascular etiology were examined. All the examined patients were under dispensary observation at the Republican Specialized Scientific and Practical Medical Center of Nephrology and Kidney Transplantation of the Ministry of Health. The average age of the patients was 41.3+14.7 years.The diagnosis of CKD was established in accordance with the criteria of KDIGO (2012) [16]. Stage 2: GFR 89-60 ml/min/1,73m2 (n=35 patients), stage 3: GFR 59-30 ml/min/1,73m2 (n=33 patients with stage C3a and n=37 patients with stage C3b CKD). The assessment of the functional state of the kidneys was carried out on the basis of determining the level of serum creatinine (Cr), albumin excretion in urine (determination of microalbuminuria (MAU ≥300 mg/l) in single morning urine, glomerular filtration rate (GFR) calculated according to the EPI GFR formula, which takes into account race, gender, age, serum creatinine level blood [4,5,12]. To calculate the GFR using the CKD-EPI formula, special applications for mobile devices (QxMDCalculator) were used. The paraclinical spectrum also included the determination of the following biochemical markers, such as parathyroid hormone (PTH), calcium (Ca), phosphorus (P), calcium-phosphorus product (Ca×P), alkaline phosphatase (ALP), total protein, albumin, urea (on the biochemical analyzer "SIEMENS ADVIA 2400", Germany). When assessing the level of vitamin D, we were guided by the recommendations of KDOQI on the treatment of disorders of bone and mineral metabolism in patients with CKD from 2005 and on the nutrition of patients with CKD from 2008 [2,3,4,5]. According to these recommendations, we have formed variants according to the level of serum calcidiol: normal – ≥ 30 ng/ml; deficiency – 15-29 ng/ml; deficiency – ≤15 ng/ml.The exclusion criteria were: mineral and bone disorders unrelated to CKD, exacerbation of the underlying disease within 6 months, a history of kidney transplantation, a history of parathyroidectomy, rheumatic heart defects, cardiomyopathy, high-grade cardiac arrhythmias, heart failure III-IV FC, systemic connective tissue diseases, severe atherosclerosis, abuse alcohol, chronic liver disease, severe BEN.To assess the parameters of the structural and geometric parameters of the left ventricle and determine the types of remodeling, all patients underwent echocardiography with Dopplerography on the Mindray apparatus (China) by the transthoracic method in the supine position and on the left side in M- and B- modes in accordance with the recommendations of the American Association of Echocardiography (ASE). At the same time, the following were evaluated: the end-diastolic and end-systolic dimensions of LV (OCDS and OCS), the thickness of the posterior wall of LV (TPWLS) and interventricular septum (TIM), the size of the left atrium (LA) [179, 205]. The value of the mean hemodynamic AP (ARav.) was calculated using the Hickam formula. The indicators were calculated - final systolic and diastolic volumes (FCV and FPV), LV ejection fraction (LV), shock volume (SHV) - as the difference between FPV and FCV. LV myocardial mass (MMLV) was calculated according to the Devereux R.B. formula. At the same time, signs of LV hypertrophy (HLS) were considered if: TMJP >1.0 for men (>0.9 for women) and IMMLV > 115 g/m2 for men (> 95 g/m2 for women).The severity of HLS was determined by the following criteria:- Light – for men TITSLV 1,1-1,3 sm, IMMLV 116-131 g/m2 and for women TITSLV 1,0-1,2 sm, IMMLV 96-108 g/m2- Moderate – for men TITSLV 1.4-1.5 sm, IMMLV 132- 148 g/m2 and for women TITSLV 1.3-1.5 sm, IMMLV 109-121 g/m2- Heavy – for men TITSLV ≥ 1,7 sm, IMMLV ≥ 149 g/m2 and for women TITSLV ≥ 1,6 sm, IMMLV ≥ 122 g/m2≥LV remodeling types were determined by the following criteria:- normal LV geometry: normal IM LV and RLT <0.42 - concentric LV remodeling: normal IMMLV and RLT≥0.42 - concentric hypertrophy LV: IMMLV is greater than normal and RLT ≥0.42) - eccentric LV hypertrophy: IMMLV is greater than normal and RLT <0.42Statistical analysis of the results was carried out using the program "Statistica v7.0" ("StatSoft Inc", USA). Descriptive statistical methods were used to determine the frequency of occurrence of the trait, the arithmetic mean, median and standard deviation (SD) Differences in the indicators of biological markers between groups of CKD were calculated using the nonparametric Mann–Whitney criterion.
3. Research Results and Their Discussion
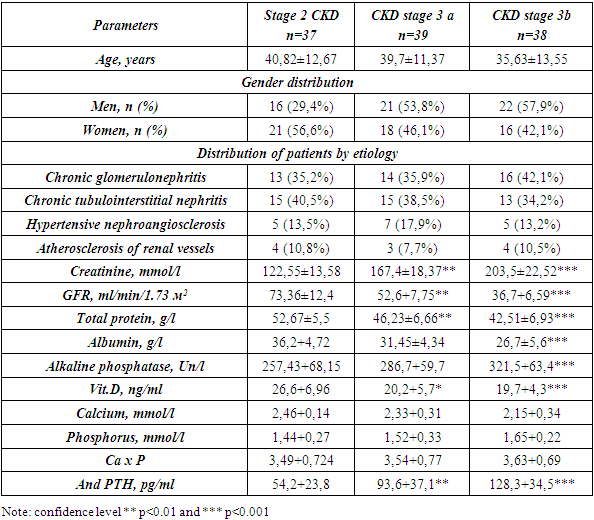
- The clinical characteristics of the examined patients with CKD with stage 2-3 are presented in Table No. 1. As can be seen from the presented data, the average age and gender composition of the examined patients was comparable. The analysis of the etiological structure of these patients showed that in all groups of patients with CKD with stages 2, 3a and 3b, patients with chronic glomerulonephritis and tubulointerstitial nephritis prevailed. The lowest percentage of the etiological factor of CKD were patients with hypertensive and atherosclerotic etiology (from 4-7%) (Table 1).Analysis of the distribution of patients with CKD depending on the level of VitD showed that as the severity of CKD increased, there was an increase in the number of patients with vitamin D deficiency and deficiency. Analysis of serum vitamin D levels revealed that 56.2% of patients had a decrease, and 26.7% of patients had vitamin D deficiency. At the same time, in group 1 with CKD C2, 22 patients (59.5%) had normal values of Vit D, then in the 2nd group of patients with CKD3a stages were 4 (10.2%), and in the 3rd group this contingent of patients was equal to 0. The number of patients with insufficient Vit. D in group 1st was detected in 3 (8.1%) patients, in group 2 in 9 (23.1%) and in group 3 with CKD stage 3 in 17 (44.7%) patients (Table 1).
|
4. Conclusions
- Our study revealed that patients with CKD and vitamin D deficiency had significantly lower serum albumin levels. However, the question remains about the determination of vitamin D in patients with severe stages of CKD and in need of hemodialysis, since the effect of dialysis on bone and mineral metabolism is great and it is difficult to assess the true picture of bone and mineral metabolism in such patients. The determination of serum vitamin D and its correction in patients with CKD has been the subject of controversy due to different results obtained by different researchers [10,11,14,16]. It is proposed to determine the serum level of "nutritional" vitamin D in patients who do not receive dialysis, and to correct for its deficiency.The obtained results of the study of EchoCG parameters indicate a significant contribution of Vit.D deficiency in patients with CKD in the progression of LV remodeling. The results of the analysis of LV size indicators revealed the peculiarity of structural and geometric changes in the left heart with Vit deficiency.D in patients with CKD. Patients with CKD with Vit.D deficiency are characterized by more pronounced manifestations of cardiac remodeling and with a predominance of eccentric LVH.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML