-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2024; 14(1): 16-23
doi:10.5923/j.ajmms.20241401.04
Received: Dec. 5, 2023; Accepted: Dec. 21, 2023; Published: Jan. 3, 2024

Clinical and Immunological Characteristics of Patients with Type 2 Diabetes Mellitus Complicated by Chronic Kidney Disease that Underwent COVID-19
Najmutdinova D. K.1, Mirakhmedova Kh. T.1, Khusanov A. M.2, Kudaybergenova D. Kh.1, Nurmatov A. Kh.2
1Tashkent Medical Academy, Uzbekistan
2Zangiata Infectious Diseases Hospital №1, Uzbekistan
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Coronavirus disease—COVID-19 (coronavirus disease 2019) has become the cause of the global pandemic in the last three years. Its etiological factor is SARS-CoV-2 (Severe Acute Respiratory Syndrome Coronavirus type 2). Patients with diabetes (DM—diabetes mellitus), in contrast to healthy people not suffering from chronic diseases, are characterised by higher morbidity and mortality due to COVID-19. Patients who test positive for SARCoV-2 are at higher risk of developing hyperglycaemia. In this paper, we present, analyse and summarize the data on possible mechanisms underlying the increased susceptibility and mortality of patients with diabetes mellitus in the case of SARS-CoV-2 infection. However, further research is required to determine the optimal therapeutic management of patients with diabetes and COVID-19. Kidney impairment in hospitalized patients with SARS-CoV-2 infection is associated with increased in-hospital mortality and worse clinical evolution, raising concerns towards patients with chronic kidney disease (CKD). From a pathophysiological perspective, COVID-19 is characterized by an overproduction of inflammatory cytokines (IL-6, TNF-alpha), causing systemic inflammation and hypercoagulability, and multiple organ dysfunction syndrome.
Keywords: COVID-19, Type 2 diabetes mellitus, IL11, IL 17A, TGF-β1
Cite this paper: Najmutdinova D. K., Mirakhmedova Kh. T., Khusanov A. M., Kudaybergenova D. Kh., Nurmatov A. Kh., Clinical and Immunological Characteristics of Patients with Type 2 Diabetes Mellitus Complicated by Chronic Kidney Disease that Underwent COVID-19, American Journal of Medicine and Medical Sciences, Vol. 14 No. 1, 2024, pp. 16-23. doi: 10.5923/j.ajmms.20241401.04.
Article Outline
1. Introduction
- The World Health Organization (WHO) has classified the 2019 Coronavirus pandemic (COVID-19) as a global health emergency [1]. The virus causes acute respiratory distress syndrome. So far our knowledge of this disease has been limited. The new beta-coronavirus was first introduced in Wuhan, China in 2019 and then spread worldwide. SARS-CoV-2 is an RNA virus with genomes ranging from 26,000 to 32,000 base pairs [2]. The capsid contains four structural proteins: spike (S), nucleocapsid (N), membrane (M) and envelope (E). In their research, Walls et al. showed that the nucleic acid and developmental N acid are under the formed lipid novel solution of the SARS-CoV-2 virion [3]. Under an electron microscope, the S looks like a corona and thus its name: coronavirus. The S protein consists of two domains. The binding of the receptor for angiotenin-converting enzyme-type 2 (ACE-2) takes place in the upper lobular domain, which initiates a viral entry into cells. The downstream domain of the S protein is involved in the virus attachment to the host-cell membrane [4]. The function of the M protein is to bind the viral genome to the inner surface of the host-cell membrane. The C-terminal domain of the transmembrane proteins contacts the viral N protein, playing an important role in its life cycle [5]. The scheme showing the model of COVID-19 virus is presented in Figure 1.
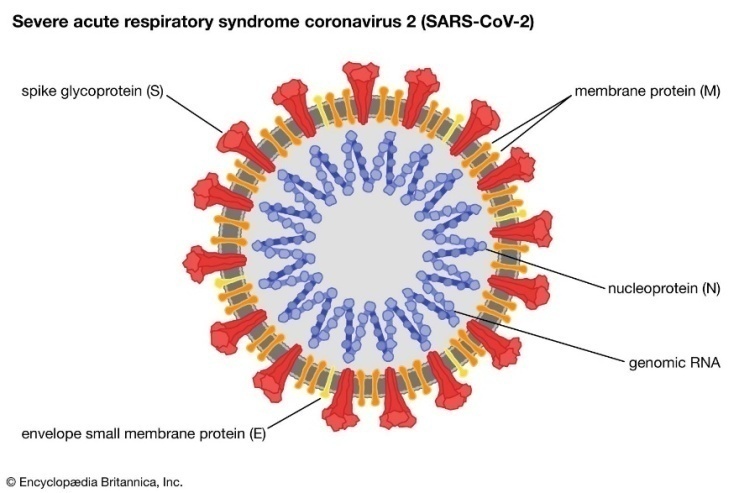 | Figure 1. A model of COVID-19 depicting structural elements of the virion |
2. Mechanism of SARS-CoV-2 Morbidity in Diabetes
- Diabetes is the seventh leading cause of death in the world and is associated with vascular complications, severely impacting quality of life(63) Diabetes predisposes patients to acquiring the infection due to an impaired immune system function. The following are responsible for this fact: the inhibition of phagocytosis, lower macrophage activity and decreased ability of neutrophil chemotaxis resulting from the reduced amount of interferon (IFN-γ) [42]. The role of glycaemic control in diabetic patients is emphasized. Although, as mentioned earlier [21], it does not seem to affect the efficacy of infection treatment, it reduces the likelihood of virus proliferation in cells [43,44]. Another possible cause of increased susceptibility to COVID-19 infection is an increase in the levels of furin, a type 1 protease bound to the cell membrane. It has been shown that higher levels of furin in diabetic patients facilitates the entry of SARS-CoV-2 into cells [33,45]. Furins mediate a viral entry into a human cell by cleaving and stimulating the SARS-CoV2 S1 and S2 proteins [3,46]. The scheme showing the pathophysiological mechanisms including an increased susceptibility of diabetic patients to COVID-19 is presented in Figure 2.
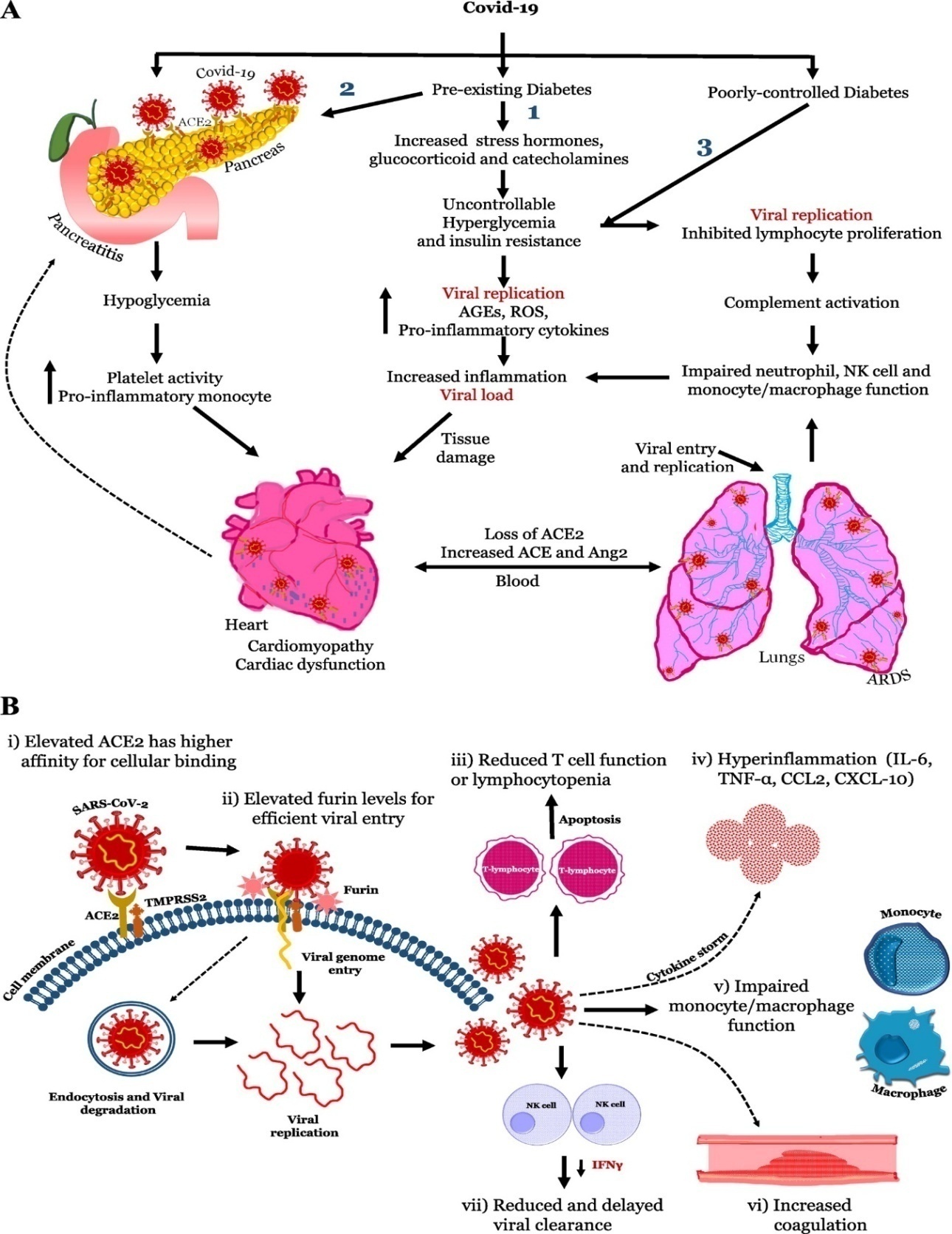 | Figure 2. Patomechanism inducing an increased possibility of COVID-19 infection in diabetic patients |
3. Inflammatory Cytokines in the Pathophysiology of Diabetic Nephropathy
- Cytokines are a group of pharmacologically active, low molecular weight polypeptides with autocrine, paracrine, and juxtacrine effects which, in a coordinated manner, regulate inflammatory and immune responses with the participation of different cytokine-associated signalling pathways. Cytokines are produced throughout the body by cells of varied embryological origin and, in addition to their immune response regulatory role, exert important pleiotropic actions as cardinal effectors of injury [36]. At present time, it is recognized that chronic low-grade inflammation and activation of the innate immune system are closely involved in the pathogenesis of diabetes mellitus [37-39]. Plasma concentrations of diverse inflammatory parameters are elevated in diabetic patients [8-10,40,41] being strong predictors of the development of this disease [42-44]. The pathogenesis of severe COVID-19 reflects an inefficient immune reaction to SARS-CoV-2. Here we analyze, at the single cell level, plasmablasts egressed into the blood to study the dynamics of adaptive immune response in COVID-19 patients requiring intensive care. Before seroconversion in response to SARS-CoV-2 spike protein, peripheral plasmablasts display a type 1 interferon-induced gene expression signature; however, following seroconversion, plasmablasts lose this signature, express instead gene signatures induced by IL-21 and TGF-β, and produce mostly IgG1 and IgA1. In the sustained immune reaction from COVID-19 patients, plasmablasts shift to the expression of IgA2, thereby reflecting an instruction by TGF-β. Despite their continued presence in the blood, plasmablasts are not found in the lungs of deceased COVID-19 patients, nor does patient IgA2 binds to the dominant antigens of SARS-CoV-2. Our results thus suggest that, in severe COVID-19, SARS-CoV-2 triggers a chronic immune reaction that is instructed by TGF-β, and is distracted from itself.of existing TGF-β inhibitors for the prevention and treatment of COVID-19 associated microvascular thrombosis and immune dysregulation.
4. TGF-β1 Promotes Renal Fibrosis
- Diabetic kidney disease pathology is characterized by thickening of the glomerular basement membrane, mesangial expansion, segmental glomerulosclerosis or global glomerulosclerosis, tubulointerstitial fibrosis, and afferent and efferent arteriole hyalinosis (Najafian et al., 2015). TGF-β is also known as a prominent regulator of immune reactions12, and it causes fibrosis13, a comorbidity of severe COVID-19 patients14,15 For a cohort of patients with severe COVID-19 requiring intensive medical care for up to 60 days, the continuous egress of plasmablasts into the blood, reflecting a continued immune reaction. Initially, within the first week of ICU admission, this immune reaction is directed against SARS-CoV-2, as all patients acquired IgG antibodies against the S and N proteins. Later, IgA-expressing plasmablasts are generated predominantly, reflecting continued instruction of the B lymphocytes by TGF-β. However, regarding their specificity, only one-third of the patients express S protein-specific IgA, and only 1 of those expressed S protein-specific IgA2, the terminal antibody class targeted by TGF-β. Circulating plasmablasts are clonally expanded and their antibodies are somatically mutated, but not specific for SARS-CoV-2 S or N protein. Taken together, these results point to TGF-β as a key cytokine regulating a chronic immune reaction in severe COVID-19, an immune reaction which is no longer directed to SARS-CoV-2.
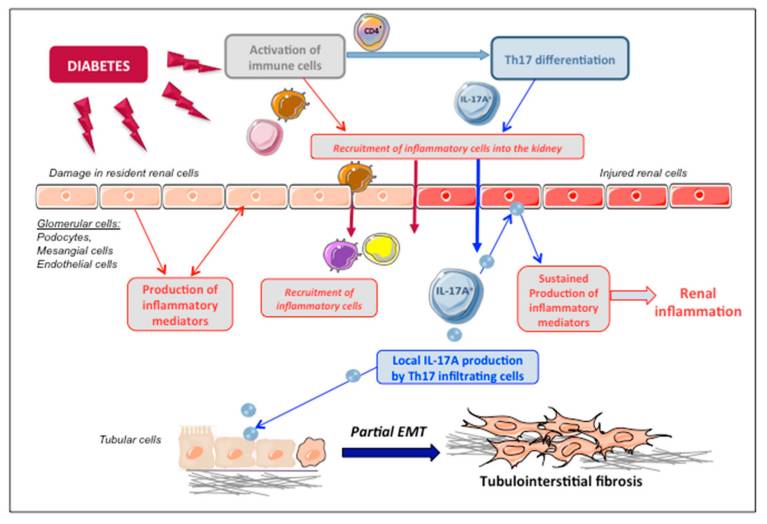
5. IL-17A as a Proinflammatory Mediator in DN
- IL-17A has been described as a circulating inflammatory protein associated with increased risk of renal damage progression [26]. Interestingly, circulating IL-17A levels are related to the severity of kidney disease and progressively decrease from subjects with normal glucose tolerance to subjects with type 2 diabetes with and without DN [40]. In agreement with this, patients with advanced DN present lower levels of IL-17A in both plasma and urine [41]. However, Zhang et al. showed an increase in CD4+ CXCR5+ PD-1+ T follicular helper cells and plasma values of IL-6 and IL-17 in patients with DN compared to healthy controls [42]. Among cirrhotic hepatitis C virus infected patients, serum IL-17A levels were higher in those that were type 2 diabetic than in non-diabetic patients and controls [43]. Although these studies have addressed circulating or urinary IL-17A levels in DN patients, local renal levels of IL-17A have not been investigated yet. Importantly, infiltration of immune cells is a key feature of DN [17]. Activated T cells (CD4+ and CD8+) are mainly located in the renal interstitium of diabetic kidneys [44,45,46]. Although CD4+ IL-17+ cells are the main source of IL-17A production, other cells, including macrophages, neutrophils, natural killer, dendritic, and mast cells, have also been described to produce this cytokine.
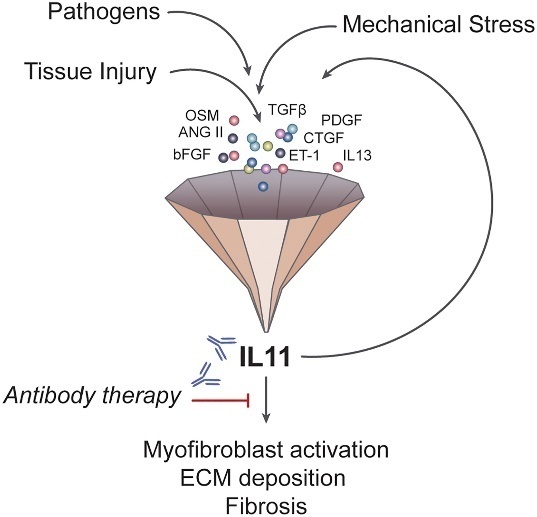
6. Cellular and Molecular Mechanisms of Fibrosis
- Interleukin (IL)-11 is a member of the IL-6 family of cytokines. Interleukin (IL)-11 is upregulated in a wide variety of fibro-inflammatory diseases such as systemic sclerosis, rheumatoid arthritis, pulmonary fibrosis, inflammatory bowel disease, kidney disease, drug-induced liver injury, and nonalcoholic steatohepatitis. IL-11 is a member of the IL-6 cytokine family and has several distinct properties that define its unique and nonredundant roles in disease. The IL-11 receptor is highly expressed on stromal, epithelial and polarized cells, where noncanonical IL-11 signaling drives the three pathologies common to all fibro-inflammatory diseases—myofibroblast activation, parenchymal cell dysfunction, and inflammation—while also inhibiting tissue regeneration. This cytokine has been little studied, and publications on IL-11 peaked in the early 1990s, when it was largely misunderstood.
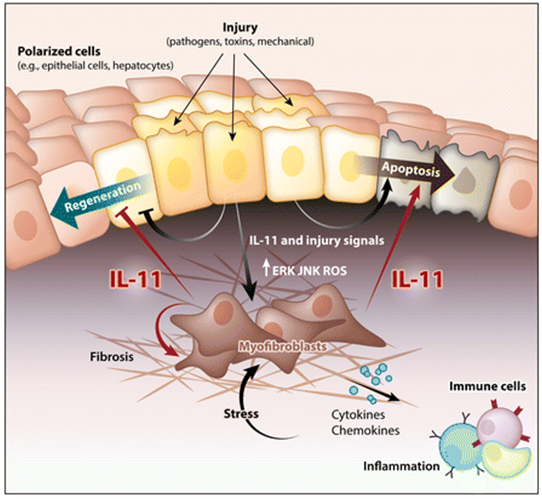 IL11 is similar to IL6, and both form a GP130 heterodimer complex to initiate its downstream signaling 25–28, but their respective hexameric signaling complex formation differ 29. While IL6R is expressed most highly on immune cells, IL11RA is expressed in stromal cells, such as fibroblasts and hepatic stellate cells, and also on parenchymal cells, including hepatocytes. Hence, it may be expected that IL6 biology relates mostly to immune functions whereas IL11 activity is more closely linked to the stromal and parenchymal biology 25, 30–32. Since the nineties, high IL11 release during viral infections have been described 33, 34, and more recently, several studies have related this interleukin to fibrosis, chronic inflammation and matrix extracellular remodeling 31, 35–39. It is also known that WNT5A and IL11 have the ability of activating STAT3 signaling 40 and this ability has been postulated as a possible mechanism to link WNT5A gene with immunomodulation. WNT5A is a member of WNT family proteins which plays critical roles in a myriad of processes in both health and disease, such as embryonic morphogenesis, fibrosis, inflammation or cancer 41. Several studies have described a crosstalk between transforming growth factor-beta (TGFβ) and WNT signaling pathways during fibrotic processes 42–45, and more recently with the increase in IL11 production 46. TGFβ represents the most prominent profibrotic cytokine by upregulating production of extracellular matrix (ECM) components and multiple signaling molecules 47.L11 signaling-related genes, such as STAT3 or TGFβ, were also differentially expressed. Subsequently, bioinformatics and functional assays revealed that these four accessory proteins were implicated in both inflammatory and fibrotic responses, suggesting the involvement of ORF6, ORF8, ORF9b and ORF9c in inflammatory and/or fibrotic responses in SARS-CoV-2 infection.
IL11 is similar to IL6, and both form a GP130 heterodimer complex to initiate its downstream signaling 25–28, but their respective hexameric signaling complex formation differ 29. While IL6R is expressed most highly on immune cells, IL11RA is expressed in stromal cells, such as fibroblasts and hepatic stellate cells, and also on parenchymal cells, including hepatocytes. Hence, it may be expected that IL6 biology relates mostly to immune functions whereas IL11 activity is more closely linked to the stromal and parenchymal biology 25, 30–32. Since the nineties, high IL11 release during viral infections have been described 33, 34, and more recently, several studies have related this interleukin to fibrosis, chronic inflammation and matrix extracellular remodeling 31, 35–39. It is also known that WNT5A and IL11 have the ability of activating STAT3 signaling 40 and this ability has been postulated as a possible mechanism to link WNT5A gene with immunomodulation. WNT5A is a member of WNT family proteins which plays critical roles in a myriad of processes in both health and disease, such as embryonic morphogenesis, fibrosis, inflammation or cancer 41. Several studies have described a crosstalk between transforming growth factor-beta (TGFβ) and WNT signaling pathways during fibrotic processes 42–45, and more recently with the increase in IL11 production 46. TGFβ represents the most prominent profibrotic cytokine by upregulating production of extracellular matrix (ECM) components and multiple signaling molecules 47.L11 signaling-related genes, such as STAT3 or TGFβ, were also differentially expressed. Subsequently, bioinformatics and functional assays revealed that these four accessory proteins were implicated in both inflammatory and fibrotic responses, suggesting the involvement of ORF6, ORF8, ORF9b and ORF9c in inflammatory and/or fibrotic responses in SARS-CoV-2 infection.7. Conclusions
- In conclusion, inflammatory cytokines could play a crucial role in the pathogenesis of DN. Monitoring of cytokines helps evaluate the immune status inflammation of DN patients and identify those patients at high risk of developing diabetes to select the best therapeutic option. Taking into account 120 patients with diabetes mellitus 2 type infected with SARS-CoV-2 in Uzbekistan, as well as a review of the literature, our observations showed that among patients complicated by diabetes mellitus, severe disease and high mortality rates are observed. Therefore, we set ourselves the task of studying this situation more deeply.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML