-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2024; 14(1): 10-15
doi:10.5923/j.ajmms.20241401.03
Received: Dec. 5, 2023; Accepted: Dec. 28, 2023; Published: Jan. 3, 2024

COVID-19 Associated Invasive Aspergillosis in Intensive Care Units: Diagnosis and Treatment
Sh. A. Tilavberdiev1, F. A. Madaminov2, E. R. Ruzibaeva2, T. X. Kadirov2, D. I. Boltaboyeva2, M. U. Boltabayev2
1Republican AIDS Control Center, Tashkent, Uzbekistan
2Fergana Medical Institute of Public Health, Fergana, Uzbekistan
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

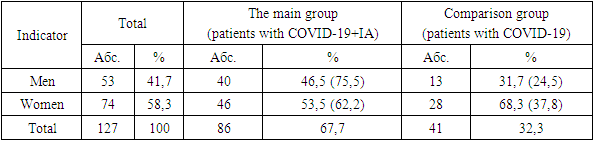
The study included 127 patients who received treatment in the intensive care unit, aged 55 years. Of these, 86 (67.7%) patients diagnosed with COVID-IA (median age-59 years, men 75%), with a positive test for the antigen aspergill galactomannan (GM), who made up the main group. The comparison group included 41 patients (32.3%) in whom IA was excluded, the median age was 57 years, men - 24%). In 90.6% of cases, patients took antibiotics, in 87.4% of cases – GCS. The high efficiency of the combination of antimycotic drugs voriconazole and Fargals was noted, which made it possible to achieve a positive result in all treated patients in the absence of fatal outcomes.
Keywords: COVID-19, Invasive aspergillosis, COVID-IA association, Risk factors, GCS, KT, Galacomannan, CD4+ lymphocytes, Voriconazole, Fargals
Cite this paper: Sh. A. Tilavberdiev, F. A. Madaminov, E. R. Ruzibaeva, T. X. Kadirov, D. I. Boltaboyeva, M. U. Boltabayev, COVID-19 Associated Invasive Aspergillosis in Intensive Care Units: Diagnosis and Treatment, American Journal of Medicine and Medical Sciences, Vol. 14 No. 1, 2024, pp. 10-15. doi: 10.5923/j.ajmms.20241401.03.
1. Introduction
- Invasive pulmonary aspergillosis (ILA) is a serious complication of severe respiratory viral infections, especially in patients in intensive care units. A large cohort of such patients was registered during the COVID-19 pandemic in 2020-2022 and the problem has become even more urgent. Coronavirus pneumonia, complicated by the addition of fungal microflora of the genus Aspergillus fumigatus, dramatically worsens the prognosis, increases the duration of treatment and the number of adverse outcomes, especially in patients with severe and critical course. This development of the disease is typical for elderly people with a weakened immune response and concomitant chronic diseases. Particular alertness is caused by patients in ICU departments with multiple organ and pronounced immune insufficiency, which is a favorable condition for opportunistic infection, primarily invasive aspergillosis (IA). According to various data, invasive lung aspergillosis associated with COVID-19 occurs on average in 2.5–15% of patients with coronavirus infection. Among patients on a ventilator — in 18-35% [11].The emergence of a new extremely dangerous pathogen in the world – the COVID-19 coronavirus (SARS-CoV-2) has led to a significant increase in competition between this virus and pathogenic fungi (Aspergillus) for the invasion of susceptible, primarily immunocompromised patients. As a result, the risk of developing associated pneumonia with a severe course and often an unfavorable prognosis has significantly increased, which necessitates a cautious attitude towards such patients [1,2,13,14,15].The entrance gate of the coronavirus is the epithelium of the upper respiratory tract and epithelial cells of the stomach and intestines. The initial stage of infection is the penetration of the pathogen into target cells having angiotensin converting enzyme type II (APF2) receptors. APF2 is localized in the cytoplasmic membrane of many types of human cells, including type II alveolar cells in the lungs, enterocytes of the small intestine, endothelial cells of arteries and veins, smooth muscle cells of arteries, macrophages in tissues of various organs [3,4,5,16].The leading manifestation of COVID-19 is pneumonia. However, such a pathological condition can be caused not only by coronavirus, but also by other microorganisms, including bacteria, viruses, fungi, protozoa. Any pneumonia is clearly visible on CT. Foci of coronavirus pneumonia during this examination look like "frosted glass". Usually it is bilateral pneumonia, most often, especially in the initial stages, in the lower parts. Along with COVID-19 in immunocompromised patients, similar lung lesions, including the "frosted glass" symptom, can also cause mycoses, primarily aspergillosis. In these conditions, diagnostic errors with severe consequences are possible. COVID-19 associations with invasive mycoses (MI) are also very likely [1,17,18].The treatment of pulmonary and generalized aspergillosis is a difficult task. Effective therapy should be comprehensive and include, in addition to the use of antifungal drugs, pathogenetic therapy, treatment of concomitant diseases, elimination and reduction of the severity of risk factors for the development of mycoses, primarily violations of local or systemic immune protection, as well as, if necessary, surgical intervention. [19,20,21,22].When prescribing and conducting antifungal therapy, it is necessary to take into account the characteristics and properties of antimycotics. Of the large number of existing antifungal drugs, only a very narrow number can be used to treat IA. The drug of choice is voriconazole, the alternatives are caspofungin, the lipid complex of amphotericin B and posaconazole [20,23,24,25].A significant barrier to adequate antifungal therapy is the risk of serious side effects and toxic effects, as well as the often high cost of antimycotics. In this regard, the search for new approaches and means to control them, improve the effectiveness of their treatment and prevention seems very relevant. In the light of the above, the study of the domestic biotechnological drug Fargals seems promising. It is a complex of metabolites of autotrophic iron-oxidizing bacteria and is characterized by a wide spectrum of antimicrobial action, including, in addition to bacteria, fungi of the genera Candida and Aspergillus, as well as viruses, a low frequency of side effects, as well as stimulation of trophic affected tissues [9,26].In pulmonary forms of aspergillosis, the mortality rate is 20-35%. In the generalized (septic) form, the prognosis is unfavorable. Without treatment, IA almost always ends in death [19,27,28,29].The purpose of this study is to study the features of clinical symptoms, as well as to determine the effectiveness of IA treatment in adult COVID-19 patients in the ICU.
2. Materials and Methods
- The study included 127 patients who received treatment in the ICU, aged 55 years. Of these, 86 (67.7%) patients diagnosed with COVID-IA (median age-59 years, men 75%), with a positive test for the antigen aspergill galactomannan (GM), who made up the main group. To analyze the risk factors of development, a study was conducted in a comparison group of 41 patients (32.3%) in whom IA was excluded (41 patients, median age-57 years, men 24%). Instrumental methods consisted of computed tomography (CT) of the chest organs in high resolution mode. Laboratory diagnostics of IA included a general blood test of patients, a biochemical blood test, a galactomannan (GM) test, immune tests for the content of CD-4+ lymphocytes and IG aspergillosis antibodies.
3. Results
- Demographic characteristics of patients of the studied groups shown in table 1.
|
|
|
|
|
4. Discussion
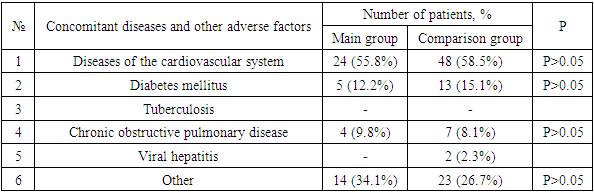
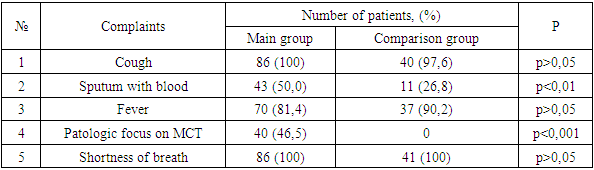
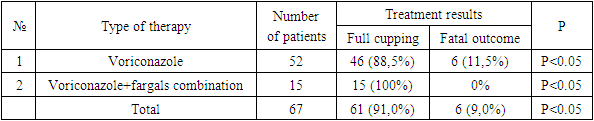
- An analysis of the literature indicates the significance and relevance of MI in immunocompromised patients, including patients with COVID-19. However, insufficient knowledge of mycoses, the complexity and complexity of diagnosis, underestimation of immunological aspects determine the inadequacy and, consequently, the low effectiveness of the therapy and high mortality. All this makes it necessary to expand and deepen scientific research in this direction.In the light of what has been said, the following unresolved and insufficiently studied issues were the motivating grounds for carrying out this work:- The role of MI, in particular IA, in COVID-19, especially in OVIR conditions;- Identification and consideration of the features of clinical manifestations and concomitant diseases, which will undoubtedly be useful in the preliminary diagnosis of mycoses;- A significant scientific contribution will be the study of regional differences in the etiological structure, clinical course and sensitivity of MI pathogens to modern antifungal agents;- Given the constant increase in the resistance of MI pathogens to antifungal drugs, as well as their insufficient availability and high cost, it seems very promising to study the antimycotic activity of the domestic biotechnological drug Fargals in vitro and in vivo;- It is important to study the effectiveness of modern methods of treatment and prevention of IA, with the inclusion of the domestic drug Fargals in the therapeutic complex;- It is relevant to develop accessible and effective recommendations for the diagnosis and treatment of IA associations with COVID-19.The study of chronobiological features of COVID-IA showed significantly more frequent detection of such patients in February, March and October compared to other months of the year, which is probably due to changes in the level of general immunity in patients. It is advisable to take this phenomenon into account when planning and conducting preventive and diagnostic measures. Analysis of the air for the presence of aspergillus in hospitals showed a relatively high contamination of clean and conditionally clean rooms with them. Such a concentration of fungal spores in the air is probably associated with high humidity and poor-quality disinfection, as well as with the lack of supply and exhaust ventilation. At the same time, the growth of aspergillus colonies was mainly detected in the air of hospital wards with aspergillosis patients, whereas such fungi were not sown in wards with patients without aspergillosis. Thus, the results obtained indicate that hospitalization of patients with COVID-19 in wards with a high content of aspergillus spores in the air contributes to the invasion of patients with these fungi and the emergence of the COVID-IA association, which undoubtedly aggravates the condition of patients and significantly worsens the prognosis.In total, we have isolated 37 strains of Aspergillus spp fungi. Of these, 9 (24.3%) cultures were identified, and 28 (75.7%) strains remained unidentified. The identified cultures were represented by: A. niger (3 strains), A. rizopus (2), A. fumigatus (2), A. fuzarium (1), A. flavus (1). All studied aspergillus cultures demonstrated high sensitivity to voriconazole and fargals when tested in vitro. This justifies the expediency of using these drugs in antimycotic therapy of patients with IA.The most important aspect of the diagnosis of opportunistic mycoses in general, and aspergillosis in particular, is its timeliness, because late diagnosis dramatically reduces the number of favorable outcomes, so often in real conditions, the appointment of therapy is based only on clinical data. The effectiveness of cultural diagnostic methods is low, in this regard, it is advisable to use methods based on the determination of antigens and metabolites of fungi, which have significantly higher sensitivity and effectiveness.Modern serological methods are aimed at indicating the unique highly immunogenic elements of the cell membrane of fungal organisms in serum or other body fluids.Detection of circulating aspergillus galactomannan (GM) antigen in serum and other body fluids (BAL, NBL) is important and makes it possible to diagnose IA at an early stage of the disease, often before the onset of clinical symptoms and the appearance of radiological signs of mycotic complication.Treatment of IA as part of the COVID-IA association is a complex and time-consuming task. We started it immediately. It included antifungal therapy and the elimination or reduction of the severity of risk factors.The drug of choice for COVID-IA therapy was voriconazole (intravenously 2x6 mg / kg on day 1, then 2x4 mg / kg/ on day). The duration of antifungal therapy was 4-6 weeks. Along with voriconazole, the domestic drug Fargals was also used inhaled with a nebulizer as part of the complex treatment. The high efficiency of the proposed combination of antimycotic drugs (voriconazole+Fargals), which made it possible to achieve the relief of the pathological process in all 15 treated patients in the complete absence of fatal outcomes. Whereas with voriconazole monotherapy, deaths were recorded in 6 (11.5%) cases (p <0.001).In the treatment of patients with IA by the combined method, positive dynamics was observed from the 2nd day in the form of a drop in body temperature, a decrease in symptoms of hemoptysis, an increase in saturation to 80-85%, and already on the 4th day the saturation level reached the norm of 90-95% (against the background of active oxygenation). On the 8th-9th day of therapy, patients switched to independent breathing with saturation up to 95-96%. The terms of treatment of patients have been significantly reduced.The effectiveness of the therapy can also be judged by the dynamics of changes in the content of CD4 lymphocytes during antimycotic therapy. At the same time, there was a significant increase in the level of lymphocytes in patients in both groups observed, which indicates an increase in their immune status.The presented results indicate that patients with COVID-19 may develop lung IA during therapy with systemic glucocorticosteroids (GCS). If COVID-IA association is suspected, it is necessary to conduct an MSCT examination of the chest organs and a mycological examination. The most effective diagnostic method is a specific galactomannan (GM) test. A significantly high efficiency of the combination of antimycotic drugs voriconazole and Fargals was established, which made it possible to achieve early clinical improvement, as well as relief of the pathological process in all treated patients in the absence of fatal outcomes.Thus, in patients in the ICU, the frequency of COVID-19 associations with IA reaches 67.7%. In this case, the addition of IA occurs against the background of severe lymphocytopenia (CD4 <350), as well as due to high doses of steroids, antibiotics and the presence of concomitant diseases. In the absence of timely diagnosis and treatment of IA, the mortality rate is 100%. The determination of GM in biological substrates (BAL, aspirate, sputum, serum) makes it possible to diagnose IA at an early stage of the disease, often before the onset of clinical symptoms and the appearance of radiological signs. Based on modern literature data and the results of our own research, we have developed effective algorithms for the diagnosis and treatment of IA in patients with COVID-19. Their use made it possible for the first time in Uzbekistan to determine the reliable frequency of opportunistic invasive aspergillosis in COVID-19 (67.7%) in immunocompromised patients at risk and undergoing inpatient treatment in the ICU of the Fergana region during a comprehensive examination using modern serological methods. This indicates the need for wider implementation of the developed algorithms in the practice of specialized institutions and an extremely cautious attitude to mycotic complications in this contingent of patients, their timely diagnosis, strengthening of preventive measures and urgent implementation of adequate etiotropic therapy.
5. Conclusions
- 1. Patients with COVID-19 on the background of therapy with systemic glucocorticosteroids (GCS) may develop invasive pulmonary aspergillosis (IA). If COVID-IA association is suspected, it is necessary to conduct an MSCT examination of the chest organs and a mycological examination. The most effective diagnostic method is the galactomannan (GM) test. 2. A positive test for GM was detected in 86 (67.7%) patients who made up the main group (COVID-IA). The comparison group included 41 patients (32.3%) in whom IA was excluded (the GM test was negative). 3. The high efficiency of the combination of antimycotic drugs voriconazole and fargals was established, which made it possible to achieve relief of the pathological process in all treated patients in the absence of fatal outcomes.Conclusion Factors predisposing to the development of IA include malignant hematological diseases, transplantation, therapy with GCS and immunosuppressants, HIV infection and some primary immunodeficiency. During the pandemic of a new coronavirus infection, patients with severe COVID-19 in the ICU entered the risk group. Due to the lack of uniform diagnostic criteria and classification of COVID-IA, data on the frequency of this fungal complication differed greatly in different countries. In 2020, the European Confederation of Medical Mycology (ECMM) and the International Society of Medical and Veterinary Mycology (Isam) proposed and developed criteria for the diagnosis of COVID-IA [12], which led to a slight decrease in its prevalence. Finally, long-distance researchers have indicated what happens with a high risk of COVID-AND in patients with COVID-19 in the ICU. Just as in the case of the COVID-IA spread schedule, the age of more than 50 years and high body weight, male sex, the use of GCS before and during treatment in the ICU, dexamethasone, diabetes mellitus, cardiovascular insufficiency, long-term treatment in the ICU, prolonged IV.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML