-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2023; 13(12): 1980-1984
doi:10.5923/j.ajmms.20231312.34
Received: Nov. 25, 2023; Accepted: Dec. 13, 2023; Published: Dec. 20, 2023

Effects of Dapagliflozin on Cardiac Functional Status in Patients with Chronic Heart Failure
Abdigaffar G. Gadaev1, Guzel B. Shamsutdinova2, Oybek Z. Abdukholikov1
1Tashkent Medical Academy, Uzbekistan
2Fergana Public Health Medical Institute, Uzbekistan
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

In the article, the effects of standard medical treatments with different content on intracardiac hemodynamics and N-pro natriuretic peptide in patients with chronic heart failure were studied by comparing the subjects in three groups. A highly reliable increase in left ventricular end-diastolic size and diastolic volume and ejection fraction was found in the third group of patients. This confirms that sacubitril+valsartan and dapagliflozin have a high cardioprotective effect.
Keywords: Chronic heart failure, N-pro natriuretic peptide, Succubitril+valsartan, Dapagliflozin
Cite this paper: Abdigaffar G. Gadaev, Guzel B. Shamsutdinova, Oybek Z. Abdukholikov, Effects of Dapagliflozin on Cardiac Functional Status in Patients with Chronic Heart Failure, American Journal of Medicine and Medical Sciences, Vol. 13 No. 12, 2023, pp. 1980-1984. doi: 10.5923/j.ajmms.20231312.34.
Article Outline
1. Introduction
- Chronic heart failure (CHF) is one of the urgent medical and social problems of modern medicine [19,12,15]. This is due to its prevalence, severe consequences, and high cost of treatment [14,4,10].Mortality due to CHF is 4-8 times higher than in the general population, and half of patients die within 5 years of diagnosis. Its IV functional class (FC) has a half-year mortality rate of 44% [21,24,1,11,17,16].According to epidemiological data, in the Russian Federation and European countries, in most cases, CHF develops as a result of arterial hypertension (95%) and ischemic heart disease (IHD) (69.7%). In our republic, the main cause of this serious complication is often the two diseases listed above [18,2].Due to the increase in the life expectancy of the population, the positive results achieved in the treatment of cardiovascular diseases, and the prevalence of risk factors that cause IHD and hypertension (HK), which are the main diseases that cause CHF, this serious complication is becoming more and more common among the world’s population [3,5,22,23]. Despite the progress made in recent years, this confirms that CHF still remains a heavy financial burden on the health economy of all countries around the world.Systemic changes are observed in all organs of CHF, and remodeling processes in the heart are of particular importance [20]. It is known that a number of examination methods are used in the diagnosis and evaluation of the effectiveness of treatment of CHF. Among them, natriuretic hormones are of particular importance as a biological marker. Currently, there are a number of its representatives, among which brain and N-pro brain sodium uretic peptides are widely used in the diagnosis and evaluation of CHF.A.M. Richards was the first to show the use of concentration of N-prosodium uretic peptide in blood to control the effect of treatment in patients suffering from CHF. In it, monitoring was carried out by titrating the dose of angiotensin-converting enzyme inhibitors (ACEI) in patients diagnosed with CHF II-III FC under hormonal control, and the appropriateness of such an approach was shown [13].In the IMPRESS trial, which included 573 patients receiving lisinopril and omapatrilat, those with CHF and left ventricular ejection fraction less than 40% had reliable reductions in neurohormone 1-2 years after initiation of treatment in a randomized trial [8].Similar data were obtained in experimental observations conducted by S. Tang and co-authors on patients receiving valsartan and benazepril [16].Also, in a series of observations, a decrease in brain sodium uretic peptide in the blood was found in patients taking β-blockers [7]. Taking into account the above, we studied N pro brain sodium uretic hormone and cardiac hemodynamics in our patients before and after treatment with different components.
2. The Purpose of the Study
- Studying the effects of various treatments on serum N pro brain sodium uretic hormone and cardiac functional status in patients with chronic heart failure.
3. Research Materials and Methods
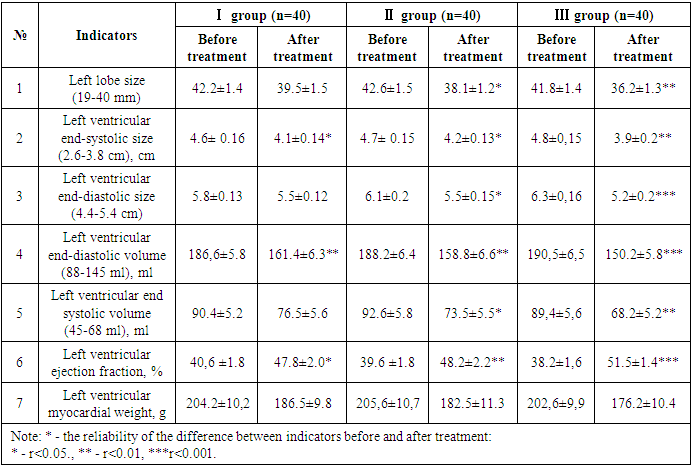
- This scientific research work was conducted in 2022 and 2023 in Fergana Public Health Medical Institute and private clinic “Farovon” in 120 patients with developed CHF on the basis of UIK and arterial hypertension (AG). They, in turn, were divided into three groups based on the treatment procedures. Each group consisted of 40 patients, 20 of which consisted of CHF II and III FC. The average age of the first group of patients was 66.1±1.8, men were 21 (52.5%) and women were 19 (47.5%). In the 1st group of patients under observation, the number of those who underwent myocardial infarction (MI) - 28 (70%), those who underwent aortic coronary bypass surgery (ACS) or stenting - 11 (27.5%), rhythm disturbances and blockades were recorded - 12 (30%), AG there were 31 (77.5%), 17 (42.5%) people with various degrees of obesity, and 21 (52.5%) people with anemia. This group was prescribed β-blockers + AAFIs or angiotensin receptor blockers (ARBs) + mineralocorticoid receptor antagonists (MRKA)-veroshpirone as the standard treatment for CHF. The average age of the second group of patients was 65.9±1.5, men were 24 (60%) and women were 16 (40%). In this group, the number of those who underwent MI - 25 (62.5%), those who underwent ACS or stenting - 13 (32.5%), those who had rhythm disturbances and blockades - 15 (37.5%), those who had AG 27 (67.5%), those who were found to be obese in various degrees - 16 (40%), anemia was observed - 19 (47.5%) people. They received a standard treatment consisting of β-blockers + succubitril-valsartan (yuperio) + MRKA-veroshpirone. The average age of the third group of patients was 64.7±1.3, 21 (52.5%) of them were men and 19 (47.5%) were women. In this group, the number of those who underwent MI - 27 (67.5%), those who underwent aortic coronary bypass surgery or stenting - 16 (40%), those who had rhythm disorders and blockades - 17 (42.5%), those who had AF 25 (62.5%), of various degrees 15 (37.5%) were diagnosed with obesity, 19 (47.5%) were anemic. β-blockers + succubitril-valsartan (yuperio) + MRKA-veroshpiron + glucose-sodium co-transporter type 2 inhibitors (dapagliflozin/forsiga) were recommended to these patients.Particular attention was paid to the same number of patients in II and III FC in each group and their representativeness.In addition to routine laboratory tests, serum N-pro natriuretic peptide indicators were determined in all subjects enrolled in the study, and cardiac functional status was assessed using EXOKG.The amount of N-pro-natriuretic peptide in blood serum was determined using immunoenzyme analysis using “Vector-BEST” (Russia) reagents. The reagent used for the determination of N-pro natriuretic peptide in the blood serum in the study had a detection range of 0-2500 pg/ml and a sensitivity of 20.0 pg/ml. Analysis and discussion of research results. We studied N-pro-brain sodium uretic peptide indicators in blood serum in all groups of patients under our observation before and after treatments. Figure 1 below shows a comparative analysis of pre- and post-treatment serum levels of N-pro brain natriuretic peptide.
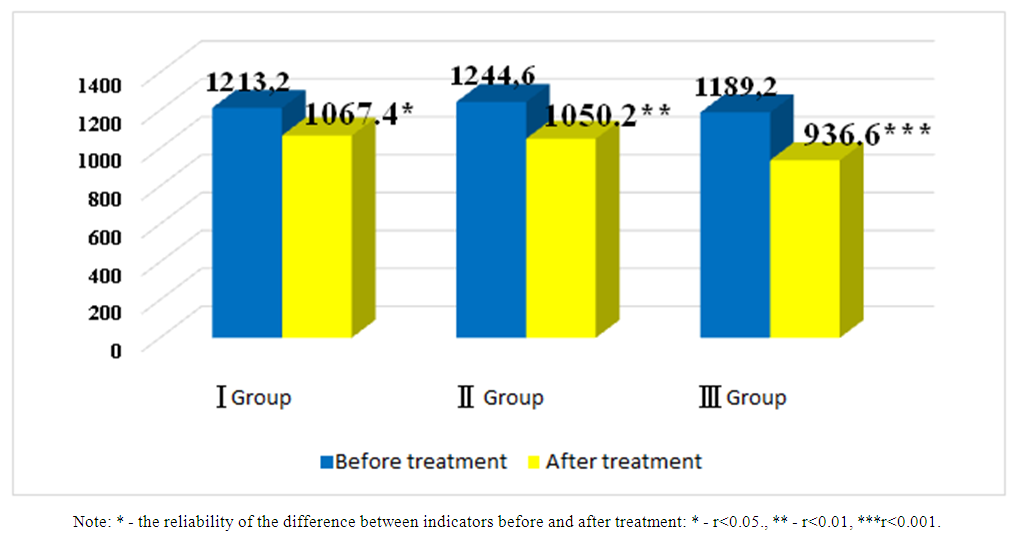 | Figure 1. Comparative analysis of pre- and post-treatment N-pro brain sodium uretic peptide indicators in patients with chronic heart failure (pg/ml) |
|
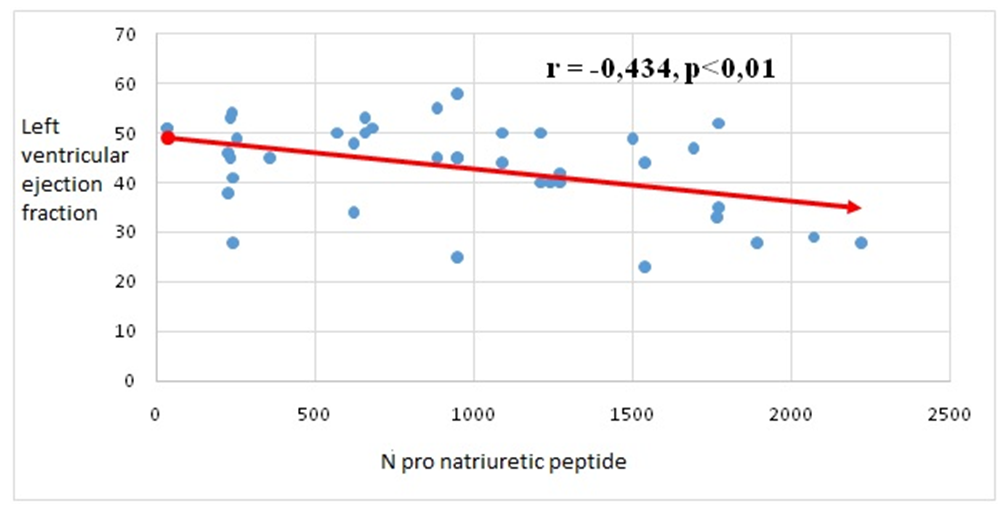 | Figure 2. Correlation between N-pro natriuretic peptide and left ventricular ejection fraction in patients with chronic heart failure |
4. Conclusions
- After treatments, positive changes in N-pro natriuretic peptide indicators were observed in all groups of patients. But in the third group, changes in its indicators at a highly reliable level confirm the effect of sacubitril + valsartan and dapagliflozin (Forsiga) on the functional state of the heart compared to the first two groups. Also, highly reliable positive changes in systolic and diastolic volume and left ventricular ejection fraction were observed in the group containing glucose-sodium co-transporter type 2 inhibitors (dapagliflozin), which showed that it is important in stabilizing cardiac hemodynamics.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML