-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2023; 13(12): 1915-1919
doi:10.5923/j.ajmms.20231312.21
Received: Nov. 13, 2023; Accepted: Dec. 1, 2023; Published: Dec. 13, 2023

Assessment of Immunologic Factors in Synovial Fluid Among Rheumatoid Arthritis Patients
Pardayev B. B.1, Ziyadullayev Sh. X2, Eshmuratov S.2, Xudoyberdiyev Sh. Sh.3
1Samarkand Branch of Republican Specialized Scientific Centre of Traumatology and Orthopedics, Samarkand, Uzbekistan
2Department of Internal Medicine 1, Samarkand State Medical University, Samarkand, Uzbekistan
3Medical School, Central Asian University, Tashkent, Uzbekistan
Correspondence to: Pardayev B. B., Samarkand Branch of Republican Specialized Scientific Centre of Traumatology and Orthopedics, Samarkand, Uzbekistan.
| Email: |  |
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Synovial fluid is a clear, viscous, straw-colored fluid in the synovial cavity of a joint that has biomechanical, met-abolic, and regulatory functions. RA synovial fluid showed a greenish, turbid appearance with decreased viscosity, alongside increased protein and glucose levels, indicative of active inflammation and altered joint metabolism. Elevated leukocytes and specific lymphocyte subpopulations (CD3+, CD8+, CD16+, CD20+, CD95+) point to a robust immune response and enhanced apoptosis in RA joints. Humoral Immunity: Marked increases in immunoglobulins (IgG, IgM, IgA) suggest significant B-cell activity, contributing to RA's chronic inflammation. Radiologic Stage Correlation: Immunologic responses in RA vary across radiologic stages, with intensified immune activity in early stages and increased apoptosis in advanced stages. These findings emphasize the value of synovial fluid analysis in RA diagnosis, progression monitoring, and treatment guidance, offering potential for targeted therapeutic strategies.
Keywords: RA, Rheumatoid arthritis, Synovial fluid, Inflammatory activity
Cite this paper: Pardayev B. B., Ziyadullayev Sh. X, Eshmuratov S., Xudoyberdiyev Sh. Sh., Assessment of Immunologic Factors in Synovial Fluid Among Rheumatoid Arthritis Patients, American Journal of Medicine and Medical Sciences, Vol. 13 No. 12, 2023, pp. 1915-1919. doi: 10.5923/j.ajmms.20231312.21.
Article Outline
1. Introduction
- Synovial fluid is a clear, viscous, straw-colored fluid in the synovial cavity of a joint that has biomechanical, metabolic, and regulatory functions. The molecular and cellular components of SF endow it with unique properties and functions to maintain joint homeostasis and frictionless movement. SF is produced by the cells of the synovial membrane, is rich in hyaluronic acid, and is infiltrated from the superficial capillaries and venules along with low molecular weight proteins. In a healthy joint, the SF acts as a medium for transporting essential nutrients, enzymes, cytokines and growth factors responsible for cartilage remodeling. The volume of synovial fluid normally depends on the size of the joint. Its maximum volume in normal knee and hip joints reaches 3.5 ml. In inflammatory processes, the volume of SF often increases, but even with a normal amount of joint fluid can not exclude the pathology of the joint. Synovial fluid contains immune cells, including mast cells, monocytes, synovial membrane cells and inflammatory cytokines, which together reflect the degree of inflammation in the affected area [2,5,9]. In musculoskeletal disorders such as RA and OA, inflammatory markers and nerve signal transducing substances have been found in the CSF samples of patients with knee OA. Sohn D.H. et al. identified 108 proteins of the SL in OA, including plasma proteins, serine protease inhibitors, proteins indicative of cartilage renewal, and proteins involved in inflammation and immunity [3,4-7]. Synovial fluid has been shown to be a useful substrate in the diagnosis of joint disease [1,6-9].In the present study, using synovial fluid samples from patients with RA, we investigated the features of immunologic parameters and interleukin profile of the intra-articular process.
2. Methods
2.1. Study Population and Methods
- This observational study was conducted to assess the physicochemical and immunological characteristics of synovial fluid in patients with rheumatoid arthritis (RA) compared to a control group with posttraumatic hemarthrosis.The study protocol was approved by the Institutional Review Board of Samarkand State Medical University. All procedures were carried out in accordance with the ethical standards of the Helsinki Declaration. Written informed consent was obtained from each participant.The RA group comprised patients diagnosed based on the American College of Rheumatology/European League Against Rheumatism classification criteria for RA. The control group included patients who had experienced joint trauma without any autoimmune disorder. Demographic data, including age, sex, and disease duration, were recorded.Synovial fluid samples were aspirated from the knee joints using sterile techniques. Samples were centrifuged to remove cellular debris and either analyzed immediately or stored at -80°C for subsequent assays.Visual Inspection: The color of the synovial fluid was noted immediately after aspiration.Viscosity Measurement: A rotational viscometer was used, calibrated before each measurement session.Biochemical Profiling: Total protein was measured using the Bradford assay, and glucose levels were determined by an enzymatic colorimetric method.Leukocyte and erythrocyte counts were performed using a light microscope and a Neubauer counting chamber. Differential cell counts were conducted to identify and quantify rhegocytes.Lymphocyte Subpopulations: Flow cytometry was used to identify CD3+, CD4+, CD8+, CD16+, CD20+, CD25+, and CD95+ lymphocytes. Fluorochrome-conjugated antibodies specific to these markers were used.Autoantibodies and Acute Phase Reactants: RF and CRP levels were quantified using high-sensitivity ELISA kits.Immunoglobulin QuantificationIgA, IgM, and IgG levels in synovial fluid were measured using sandwich ELISA techniques. Standards and samples were run in duplicate to ensure accuracy.Radiographic AnalysisJoint radiographs of RA patients were taken and classified into stages according to established radiographic criteria. The presence and extent of joint erosions and other changes were recorded.
2.2. Statistical Analyses
- In this prospective observational study we utilized descriptive statistics for an overview of the study population's demographic and clinical characteristics. We calculated means, medians, and proportions to highlight baseline features and disease duration.For the longitudinal aspect, repeated measures analyses (mixed-effects models) assessed changes in disease activity and patient outcomes over time. Survival analysis, employing Kaplan-Meier curves and Cox proportional hazards models, provided insights into the duration until events like treatment response or disease flare.Subgroup analyses explored the heterogeneity within the RA population, comparing outcomes based on factors such as seropositivity or treatment regimens. Correlation and regression analyses identified relationships between variables and predictors of outcomes, adjusting for confounders.Handling missing data involved multiple imputation techniques, ensuring robustness with sensitivity analyses. Reputable statistical software (R-studio) validated the analyses for accuracy.
3. Results
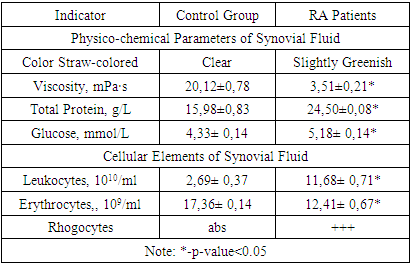
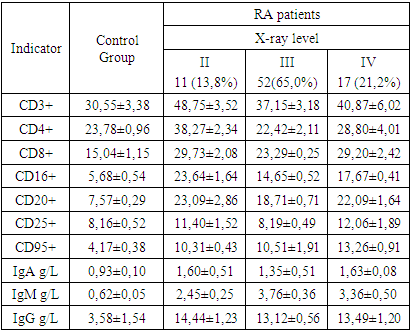
- In RA patients, the synovial fluid predominantly exhibited a slightly greenish and moderately turbid appearance, deviating from the clear, straw-colored fluid seen in the control group. This change in coloration and clarity may indicate the presence of inflammatory processes within the joint. Additionally, a significant reduction in the viscosity of the synovial fluid was observed in RA patients (3.51±0.21 mPa·s) compared to the control group (20.12±0.78 mPa·s), a change that reflects a fundamental alteration in the fluid's composition and is likely attributed to the inflammatory environment in RA-affected joints (table 1).
|
|
|
|
|
4. Conclusions
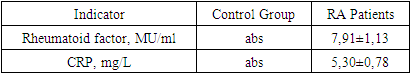
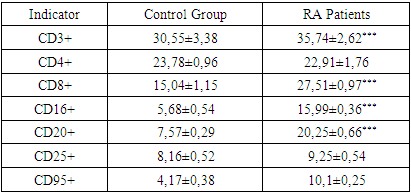
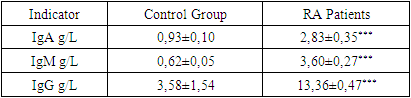
- Our comprehensive study of synovial fluid in patients with rheumatoid arthritis (RA) has yielded significant insights into the disease's pathophysiology. RA patients exhibited notable changes in the synovial fluid, including a slightly greenish and moderately turbid appearance, and a significant reduction in viscosity. These changes, coupled with increased total protein and glucose concentrations, underscore the presence of an active inflammatory process and altered metabolic activity within RA-affected joints. A marked increase in leukocytes, along with the presence of rhogocytes, indicates a robust immune response. The substantial elevation in lymphocyte subpopulations such as CD3+, CD8+, CD16+, and CD20+ cells reflects a heightened T-cell mediated and innate immune response. The increased levels of CD95+ lymphocytes highlight enhanced apoptotic activity, a critical aspect of RA pathogenesis.The significant rise in immunoglobulin levels (IgG, IgM, IgA) in RA patients points to active B-lymphocyte involvement. This finding suggests that B-cell activation and differentiation play a crucial role in RA, contributing to the chronic inflammatory state.The immune response in RA varies with the radiologic stages. In early stages, there is an intensified immune response, whereas in more advanced stages, a shift towards increased apoptosis readiness and T-cell activation is observed. This progression highlights the dynamic nature of immune involvement in RA.These findings emphasize the importance of synovial fluid analysis in diagnosing RA, monitoring its progression, and guiding treatment strategies. The distinct immunological patterns across different radiologic stages of RA can serve as valuable biomarkers for disease progression and response to therapy. This understanding can aid in the development of targeted treatments, potentially slowing the progression of joint deformities and improving patient outcomes.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML