-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2023; 13(12): 1904-1907
doi:10.5923/j.ajmms.20231312.18
Received: Nov. 8, 2023; Accepted: Dec. 5, 2023; Published: Dec. 8, 2023

Morpho-Functional Changes in Neurons of Spinal Nodes after Experimental Cholecystectomy in Mongrel Dogs
Rakhmonova Habiba Nurullayevna1, Rakhmonov Zafarjon Mamadiyevich2, Sultanbayev Shaxboz Axmadjonovich3, Rakhmonov Fariz Zafarjonovich4
1Assistant of the Department of Histology, Cytology and Embryology, Samarkand State Medical University, Uzbekistan
2Assistant of the Department of Human Anatomy, Samarkand State Medical University, Uzbekistan
3Student of the Medical-Pedagogical Faculty, Samarkand State Medical University, Uzbekistan
4Student of the Faculty of Pediatrics, Samarkand State Medical University, Uzbekistan
Correspondence to: Rakhmonova Habiba Nurullayevna, Assistant of the Department of Histology, Cytology and Embryology, Samarkand State Medical University, Uzbekistan.
| Email: |  |
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

In the experiment on mongrel dogs, we studied structural and functional changes of neurons of spinal nodes arising at experimental cholecystectomy. We described the states of neurons in control groups of dogs and in experimental cholecystectomy. Differences in the size indices and the ratio of cells with reactive and destructive changes were found between the two: control and experimental groups of animals.
Keywords: Spinal nodes, Neurons, Morphology, Cholecystectomy, Reactive and destructive changes of neurons
Cite this paper: Rakhmonova Habiba Nurullayevna, Rakhmonov Zafarjon Mamadiyevich, Sultanbayev Shaxboz Axmadjonovich, Rakhmonov Fariz Zafarjonovich, Morpho-Functional Changes in Neurons of Spinal Nodes after Experimental Cholecystectomy in Mongrel Dogs, American Journal of Medicine and Medical Sciences, Vol. 13 No. 12, 2023, pp. 1904-1907. doi: 10.5923/j.ajmms.20231312.18.
Article Outline
1. Introduction
- The study of mechanisms of compensatory-adaptive reactions of spinal nodes neurons is one of the urgent problems of modern morphology, since spinal ganglia are primary afferent centers occupying a borderline position between the central and peripheral nervous system. Disruption of the structure and function of receptor neurons forming sensitive ganglia can aggravate the course of the underlying disease and is a condition for inflammatory complications [1,2,3]. In the available literature there are practically no data on the reaction of spinal neurons to cholecystectomy. The aim of the present study was to investigate the morpho functional state of ThVII - ThX spinal node neurons (SNN) in the dynamics of experimental cholecystectomy [4,5].
2. Materials and Methods of Research
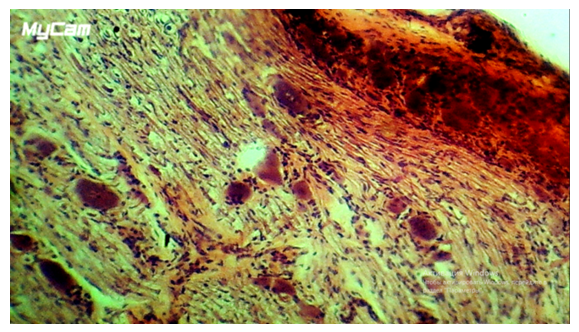
- The work was performed on 35 male adult mongrel dogs weighing 3100-3750 g. The animals were kept in individual cages in vivarium conditions with free access to water and food, on a standard diet in accordance with the norms of keeping laboratory animals. Cholecystectomy was performed under sterile conditions. Three groups of animals were formed: a group of control (C) and two experimental groups (early and late terms after surgical intervention). Morpho functional features of neurons in the first experimental group were studied in early terms after surgical interventions, in the second group of animals in late terms. To take experimental material the animals were anaesthetized with xylazine and decapitated. Animals were removed from the experiment on the 1st, 3rd, 5th, 7th, 14th, 28th day in equal groups of 5 animals each, including the control group. Thoracic ganglia ThVII - ThX were dissected as corresponding to the nerves innervating the gallbladder area. The taken biological material was fixed in Carnoua mixture and poured into paraffin mixture according to the standard technique, then 6 µm thick slices were obtained on a microtome. The obtained slices were stained with haemotoxylin-eosin and according to Nissl’s method. The studies were carried out at the light-optical level, N-300M binocular microscope with a digital camera Quality resolution of 500 megapixel was used, then the obtained images were processed using the programmer ImageJ Ver. 1,38x. To determine the profile field area of neurons, two cell diameters were measured in mutually perpendicular planes and calculated by the formula S=πAB, where, respectively, A is the larger and B is the smaller diameters. When assessing the state of spinal neurons, we distinguished cells with morphological signs of different functional states: neurons with no pronounced changes at the light-optical level, neurons with reactive or reversible changes, and neurons with destructive changes. The relative number of cells of the described groups was counted and compared. The obtained data were expressed as P±pi (%), where P (%) is the share of cells with the investigated characteristic in the total number of cells in the given group, pi – is the confidence interval of the sampling fraction. The presence of perinuclear chromatolysis, general hypochromia of cytoplasm, cell shrinkage and pericellular oedema were considered to be signs of reactive changes in neurons (Fig. 2). Destructively changed neurons were classified as cells with pronounced pycnosis, shriveling of the nucleus, with possible exit of the nucleus, vacuolisation, extreme hypo- or hyperchromia (Fig. 3). Statistical processing of the obtained results was performed using Stat Soft Statistic 6.0 package. Mann-Whitney test (u) was used to identify differences between groups; the results were considered reliable at pu < 0.05.
3. Results and Their Discussion
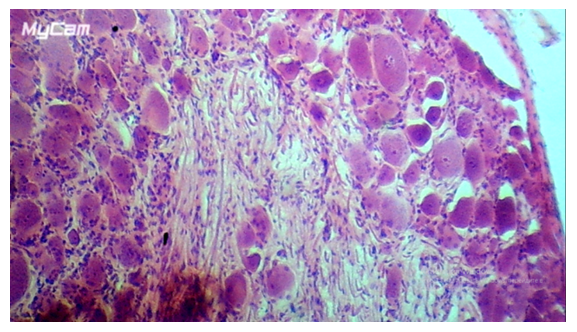
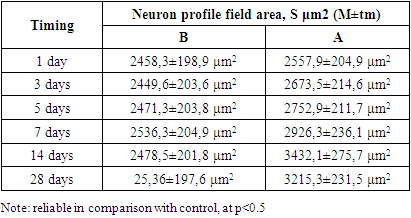
- The neurons of the spinal ganglion are represented by pseudounipolar cells, the node itself is surrounded by a connective tissue capsule. In the literature there are many classifications of spinal node neurons based on morphological features, but in most cases 3 groups of spinal node neurons are distinguished: small, medium, large [6,7]. As a result of statistical processing of morphometry data of neurons of the control group of animals, it was found that the size of pseudounipolar cells varied within the limits of 10-18 μm – small, constituted 19±1.8% of the total number of cells, 18-30 μm - medium, 49±5.8% of the total number, 30-61 μm - large neurons, 32±3.7% (Fig. 1). Among the neurons of the animals of the control group, most of them were represented by cells without signs of reactive changes (Fig. 1). In each neuron there is one nucleus of rounded shape with a well-defined nucleus. The Nissl substance was not homogeneous in its content: a part of neurons had large clumps in the perinuclear cytoplasm; in another part of cells tigroid was dispersed throughout the cytoplasm. In the spinal nodes of control animals, the proportion of neurons with signs of reactive changes in the total number was 6.4±3.5%, which, according to literature data [8,9], can be considered a manifestation of normal functional polymorphism of cells.
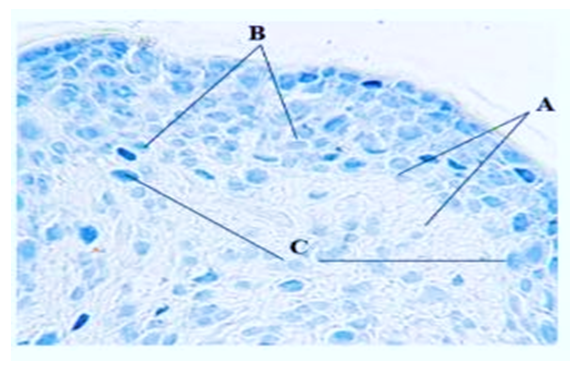 | Figure 1. Spinal node neurons in the control group of dogs. A - small cells, B - medium-sized neurons, C - large cells. Magnification x200, line size on the bottom left is 10 µm. Nissl staining |
|
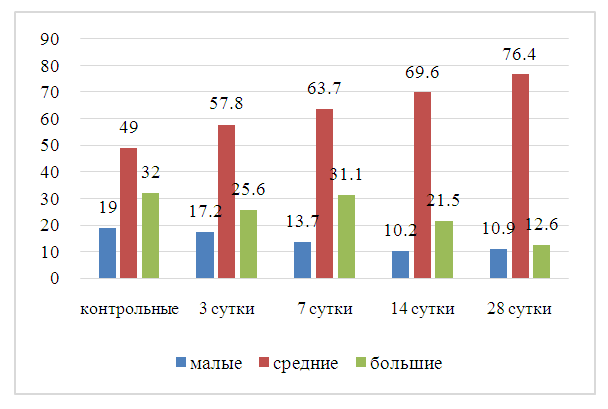 | Figure 5. Dynamics of changes in the ratios of neuron size groups in selected terms of the study after cholecystectomy. Note: reliable difference from the control group, at p<0.5 |
4. Conclusions
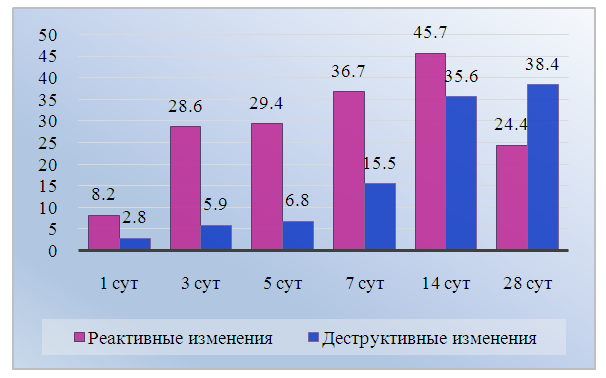
- The study of the dynamics of changes in neurons during the postoperative period reveals successive stages: 1) progressive change of the studied characteristics by 14 days of observation, reflecting the reaction of spinal neurons to cholecystectomy and the following activation of reparative processes; 2) gradual decrease of the formed disorders by 28 days, corresponding to a favorable outcome of the postoperative process. The complex of changes in spinal neurons accompanying the experimental operation includes an increase in the number of medium-sized neurons. Apparently, this is associated with multidirectional changes in the sizes of large and small neurons. The accelerated dynamics of the increase in the proportion of reactively changed neurons with the subsequent decrease in destructive changes in spinal nodes in dogs can be considered as a consequence of the activation of recovery processes. This corresponds to a more uniform and closer to the control distribution of the size groups of neurons in the experimental group by the end of the observation period.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML