-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2023; 13(12): 1833-1837
doi:10.5923/j.ajmms.20231312.02
Received: Oct. 20, 2023; Accepted: Nov. 6, 2023; Published: Dec. 2, 2023

The Molecular Basis of the Functioning of the Cytokine System and Anti-Cytokine Therapy in Rheumatoid Arthritis
Babamuradova Zarrina Bakhtiyarovna, Shodikulova Gulandom Zikriyevna
Samarkand State Medical University, Samarkand, Uzbekistan
Correspondence to: Babamuradova Zarrina Bakhtiyarovna, Samarkand State Medical University, Samarkand, Uzbekistan.
| Email: |  |
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The cytokine system is a universal, polymorphic, regulatory network of mediators designed to control the processes of proliferation, differentiation and functional activity of cellular elements in the hematopoietic, immune and other homeostatic systems of the body. One of the important issues of the pathogenesis of rheumatoid arthritis (RA) is the role of innate immunity mechanisms in the development of autoimmune inflammation. The aim of the study is to highlight the role of cytokines in the pathogenesis of RA and to improve anti-cytokine therapy. DNA samples of RA patients and healthy individuals of the therapeutic department No. 1 of the multidisciplinary clinic No. 1 of SamSMU served as the material for the study. The group of patients consisted of 49 people aged 25-45 years and a control group of 71 practically healthy individuals. It has been established that the cytokine system is a polymorphic structure and such a mechanism as allelic polymorphism is important in the formation of its polymorphism. The presented results of clinical and laboratory studies show the development of torpidity to methotrexate therapy in A subgroup of patients of both groups. Thus, the use of anti-cytokine therapy is a great achievement in the treatment of rheumatoid arthritis.
Keywords: Rheumatoid arthritis, Cytokines, Gene polymorphism, Interleukins, Tumor necrosis factor (TNFα), Monoclonal antibodies
Cite this paper: Babamuradova Zarrina Bakhtiyarovna, Shodikulova Gulandom Zikriyevna, The Molecular Basis of the Functioning of the Cytokine System and Anti-Cytokine Therapy in Rheumatoid Arthritis, American Journal of Medicine and Medical Sciences, Vol. 13 No. 12, 2023, pp. 1833-1837. doi: 10.5923/j.ajmms.20231312.02.
Article Outline
1. Introduction
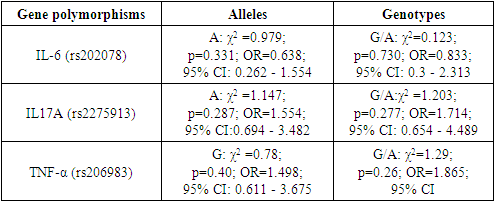
- The cytokine system is a universal, polymorphic, regulatory network of mediators designed to control the processes of proliferation, differentiation and functional activity of cellular elements in the hematopoietic, immune and other homeostatic systems of the body. Numerous studies carried out over the past 10 years have demonstrated the existence of new mechanisms for the formation of the polymorphic structure of the cytokine system [1-2,4-5]. These are allelic polymorphism of cytokine genes and alternative splicing of cytokine genes. On the one hand, these mechanisms form an even more complex polymorphic cytokine network in the body, but on the other hand, they allow us to look at its organization from a new angle.The effect of cytokines as participants in a complex network complicates the analysis of the functions of individual individual cytokines, the effect of polymorphism of their genes on the development of the immune response [8,11,19,20]. There are significant individual differences in cytokine production [6,7]. The differences between the maximum and minimum levels of production of some cytokines often reach tenfold values, and these indicators are constant at different time intervals. By studying the allelic polymorphism of genes, attempts are being made to determine the genetic basis of interindividual differences in the immune response by determining the relationship between individual polymorphic alleles, or haplotypes of cytokine genes and in vitro protein product production [12,15,23].Having studied a sufficient number of candidate genes, it is possible to identify certain genetic profiles of polymorphic cytokine genes. For example, individuals with gene variants responsible for high IFNγ production, high TNFα content and low IL-10 content have an association with inflammatory processes. Such genotypes are of functional importance, because they make it possible to explain individual susceptibility to many autoimmune, infectious diseases.Rheumatoid arthritis is a chronic systemic inflammatory disease of connective tissue with damage mainly to peripheral joints by the type of progressive symmetrical erosive-destructive polyarthritis [3], as well as characteristic extra-articular manifestations. Despite the great achievements in the study of the pathogenesis of rheumatoid arthritis (RA), which made it possible to create a fundamentally new class of fundamentally sound therapeutic agents, many immunological aspects remain not fully understood. One of the important issues of the pathogenesis of not only RA but also all rheumatic diseases is the role of innate immunity mechanisms in the development of autoimmune inflammation. Rheumatoid arthritis (RA) is the most common inflammatory joint disease, the prevalence of which in the population is about 1%, and the economic losses from RA for society are comparable to coronary heart disease. Despite the ongoing research, RA is still a disease with an unknown etiology. Moreover, there are good reasons to assume that even if it is possible to prove the role of an infectious agent in the development of some forms of RA, its elimination with the help of antibacterial or antiviral drugs is unlikely to "cure" the disease. Chronic inflammatory process leads to excessive synovial hyperplasia with proliferation of synovial cells, generation of new vessels, and diffuse or nodular infiltration by mononuclear cells [1,4,5]. The hyperplastic synovial membrane in RA is infiltrated mainly by plasma cells, dendritic cells, macrophages, which, along with synoviocytes, turned out to be the main source of "pro-inflammatory" cytokines. In addition, these cells may play a role in the presentation of local antigen to T-lymphocytes in the synovial membrane. A large number of suspected autoantigens have been described using autoantibodies present in the serum of RA patients. Despite this, there is little evidence of their involvement in the pathogenesis of RA. Antigens associated with joint tissues, such as type 2 collagen, human chondrocyte glycoprotein 39, as well as those not associated with joint tissues, such as citrulinated peptides, glucose-6–phosphate isomerase, heat shock proteins, act as antigens in RA [13]. During the immune response in RA, two closely interrelated processes occur: 1). Activation of CD4+ T-lymphocytes by Th1 type, characterized by excessive synthesis of interleukin (IL)-2, interferon-γ and IL-17; 2). Imbalance between hyperproduction of proinflammatory cytokines of predominantly macrophage nature, such as tumor necrosis factor-α (TNF-α), IL-1, IL-6, IL-8 and anti-inflammatory cytokines (IL-4, IL-10, soluble antagonist IL-1, soluble TNF-α receptors), with predominance the products of the first over the second [17]. An important role in the induction and maintenance of inflammation in the joint in RA of the proinflammatory cytokine IL-17, which is produced by CD4+ activated memory cells (CD45RO+), has been proven [24]. IL-17 stimulates the production of MMP-1 and MMP-9 and the degradation of proteoglycans, increases the expression of IL-6 and leukemia-inhibiting factor in fibroblast-like synovial cells [9,10,14,20].A more complete understanding of the mechanisms involved in the development and maintenance of rheumatoid inflammation has recently allowed the development of numerous new therapeutic approaches to its treatment. The main therapeutic task is to control the production and activity of factors involved in pathogenesis. A small part of this task was solved by drugs from the group of biological agents. For the treatment of RA, the following drugs are currently approved: Infliximab, Etanercept and Anakinra. Etanercept is a complex drug that contains two copies of the soluble recombinant TNF receptor (gp75) associated with the Fc fragment of immunoglobulin G1, etanercept blocks the biological activity of TNF by binding it, while competing with receptors on target cells.The aim of this study is to highlight the role of cytokines in the pathogenesis of RA and to improve anti-cytokine therapy.
2. Research Material and Methods
- DNA samples of RA patients and healthy individuals of the Samarkand region of the Republic of Uzbekistan served as the material for the study. The set of materials was carried out on the basis of the therapeutic department No. 1 of the multidisciplinary clinic No. 1 of SamSMU. Molecular genetic analysis was carried out in the laboratory of the RSSPMC of Hematology of the Ministry of Health of the Republic of Uzbekistan (Tashkent).The group of patients consisted of 49 people aged 25-45 years. The comparison group consisted of 71 practically healthy individuals aged 25-46 years.
3. Results and Discussions
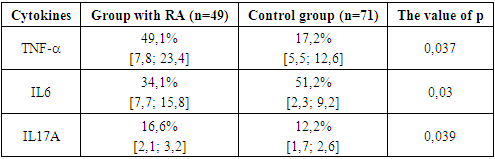
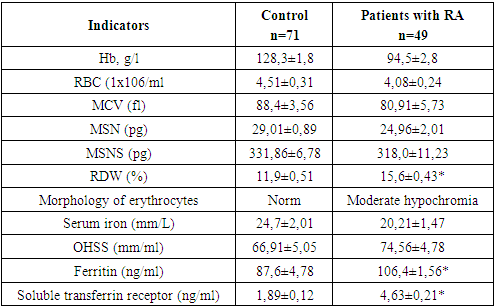
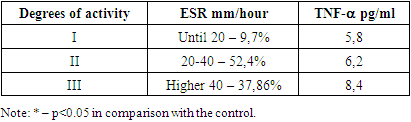
- As mentioned above, the synovial membrane of the joints in RA is infiltrated by a wide range of cells that support the immune response in the affected joint. The severity and progression of synovitis largely depends on the local interaction, activation of these cells and their release of cytokines, which in turn regulate the growth, differentiation and activation of other cells involved in inflammation and immune response in the affected joint. Local and systemic production of these cytokines is the cause of many clinical and laboratory manifestations of RA. An important place among the mechanisms of joint damage in RA is given to the so-called "pro-inflammatory" cytokines: tumor necrosis factor-α (TNF-α), interleikin-6 (IL-6) and (IL-17A). Using a variety of methodological approaches, including the use of appropriate DNA probes to assess the expression of RNA cytokines, as well as biological and immunochemical methods, it was shown that all these cytokines are synthesized in excess by synovial cells and are contained in high concentrations in synovial fluid. The studies revealed that in the group of patients with RA, the level of TNF–α was significantly increased, although to a lesser extent, but also has the ability to stimulate chondrocytes, thereby causing degradation of cartilage tissue, and also participates in bone resorption. Of fundamental importance is the fact that TNF is synthesized by cells found in excess at the junction between the pannus and articular cartilage, that is, in the area from which the destruction of the joint begins. Hyperproduction of proinflammatory cytokine IL-17 was determined in RA patients in comparison with KG. TNF-α are powerful inducers of the synthesis of another pro-inflammatory cytokine - IL-6, the concentration of which closely correlates with the clinical and laboratory parameters of the activity of the inflammatory process in RA. IL-6 is actually the only cytokine directly inducing the synthesis of acute-phase proteins by hepatocytes (Table 1).
|
|
|
|
4. Conclusions
- Pro-inflammatory cytokines play a leading role in the initiation and maintenance of the inflammatory process in the joint in RA. The increased production of their synoviocytes, mononuclear cells of peripheral blood of RA patients, has been proven by many researchers. In addition, high concentrations of the latter were found in synovial fluid and blood serum. Their main action is aimed at potentiating bone destruction, degradation of cartilage tissue by activating synovial cells, monocytes, macrophages, T- and B-lymphocytes, endothelial cells and granulocytes and their release of inflammatory mediators. Predisposition to high production of proinflammatory cytokines may be associated with inheritance of certain combinations of allelic variants of their genes. In addition, allelic polymorphism of cytokine genes affects the susceptibility to the development of RA, its severity and sensitivity to treatment. The use of anti-cytokine therapy is a great achievement in the treatment of rheumatoid arthritis.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML