-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2023; 13(11): 1798-1801
doi:10.5923/j.ajmms.20231311.41
Received: Nov. 8, 2023; Accepted: Nov. 21, 2023; Published: Nov. 25, 2023

Pathological Changes in the Wall of the Fallopian Tube in Tuboperotoneal Infertility
Shokirova S. M. 1, Israilov R. I. 2, Zufarova Sh. A. 3
1Department of Obstetrics and Gynecology No. 2, Andijan State Medical Institute, Uzbekistan
2Republican Center for Pathological Anatomy, Uzbekistan
3Republican Center for Reproductive Health, Uzbekistan
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Our work studied pathological changes in the wall of the fallopian tube in tuboperitoneal infertility. We examined surgically removed fallopian tubes during medically indicated surgery in women diagnosed with infertility. In tubal-peritoneal infertility, all layers of the wall of the fallopian tube were subjected to an inflammatory and adhesive process. Due to the inflammatory-adhesive process of the interstitium, damage to the intrinsic cell-fibrous structures of the tube wall is noted in the form of dystrophy, destruction, and degeneration, which are the morphological substrate for the non-functioning of the fallopian tube.
Keywords: Uterus, Tube, Pregnancy, Infertility
Cite this paper: Shokirova S. M. , Israilov R. I. , Zufarova Sh. A. , Pathological Changes in the Wall of the Fallopian Tube in Tuboperotoneal Infertility, American Journal of Medicine and Medical Sciences, Vol. 13 No. 11, 2023, pp. 1798-1801. doi: 10.5923/j.ajmms.20231311.41.
Article Outline
1. Introduction
- Infertility, according to various authors [1,2], occurs in 15-20% and its prevalence tends to gradually increase. In the structure of an infertile marriage, 50-60% is female infertility, among the causes of which the leading role (from 35 to 85%) belongs to the tubo-peritoneal factor and infertility in the absence of fallopian tubes [3,4,5]. Tubal-peritoneal infertility (TPI) is a consequence of the acute or chronic course of inflammatory diseases of the uterine appendages, in which it is possible to perform both radical and organ-preserving operations. Tubal and tubo-peritoneal factors of infertility (TPFI) adversely affect, on the one hand, the incidence of pregnancy using assisted reproductive technologies (ART), in particular IVF and embryo transfer into the uterine cavity (ET), on the other hand, on the number of early embryonic losses. In addition, these infertility factors often contribute to the occurrence of ectopic pregnancy. Many IVF facilities offer prior bilateral tubectomy to improve IVF outcomes [6,7]. However, many researchers [3,4,6] have confirmed the adverse effect of removal of the fallopian tubes on ovarian function, which was accompanied by menstrual irregularities, changes in ovarian function, and a decrease in ovarian reserve. In the available scientific literature there is no data on the morphological study of the wall of the fallopian tube in tuboperitoneal infertility. In this regard, this work sets the goal of studying pathomorphological changes in all layers of the wall of the fallopian tube in tubo-peritoneal infertility.
2. Material and Methods of Research
- The object of the study was biopsy material from the department of biopsy diagnostics of the Russian Medical Center, surgically removed fallopian tubes for salpingitis and infertility. After macroscopic examination, 3 pieces were cut out: the uterine part, the intermediate section, and the ampullary part of the tube. The pieces were fixed in 10% formaldehyde in phosphate buffer for 48 hours, then washed in running water. Dehydration was carried out using alcohols of increasing concentration and chloroform, and embedded in paraffin. Histological sections were stained with hematoxylin and eosin and viewed under a light microscope, and the desired areas were photographed on a computer.
3. Results of the Study and Their Discussions
- The results of a morphological study showed that with tubal-peritoneal infertility, all layers of the uterine wall are subject to an inflammatory-sclerotic process. The villi of the mucous membrane of the tube are deformed with a violation of histotopography and are presented in different shapes and sizes. The integumentary epithelium is somewhat thickened, in places metaplastic to squamous single-layer epithelium. Among the integumentary epithelium, atrophy and disappearance of the ciliated and secretory epithelium are noted; instead, intermediate cells are hyperplastic. The nucleus of the latter is hyperchromatic, relatively small and randomly located, some of them penetrate into the connective tissue plate proper. The lamina propria is expanded in area and is represented by cell-proliferative, sclerotic connective tissue (Fig. 1). The connective tissue consists of mature fibrous areas. Vessels of different sizes and shapes, their walls are thickened due to perivascular sclerosis. Study with a large microscope lens showed that the connective tissue lamina propria is dominated by mature histiocytic cells and fibrous structures. Moreover, these cells and fibrous structures are oriented around the vessels and are located parallel to the integumentary epithelium. Among the cellular composition of the connective tissue, lymphoid cells and macrophages are determined, which indicates the presence of an inflammatory process in the lamina propria (Fig. 2). Polymorphism of the integumentary epithelium or the presence in the composition of the integumentary epithelium of low round-nuclear, multirow and tall cylindrical cells. In the lamina propria of the villi of the fallopian tube mucosa, diffuse and relatively dense lymphoid infiltration is determined (Fig. 3). Lymphoid cells include small, medium and large cells and plasma cells. Due to cellular infiltration, the connective tissue is loosened in the form of disintegration of fibrous structures and swelling of the intercellular substance. The covering epithelium is flattened and desquamated over a large extent; among them there are large cells due to vacuolization of the cytoplasm.
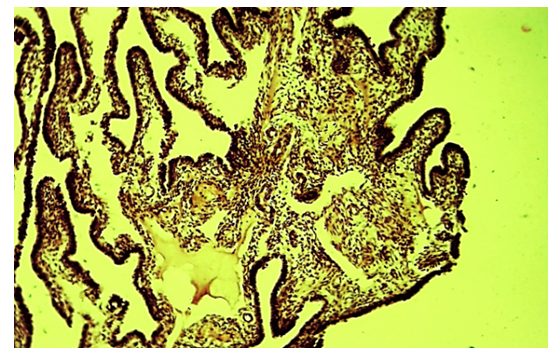 | Figure 1. Tubal-peritoneal infertility. The villi are of different shapes and sizes, the lamina propria is fibrotic and inflamed. Color: G-E. UV: 10x10 |
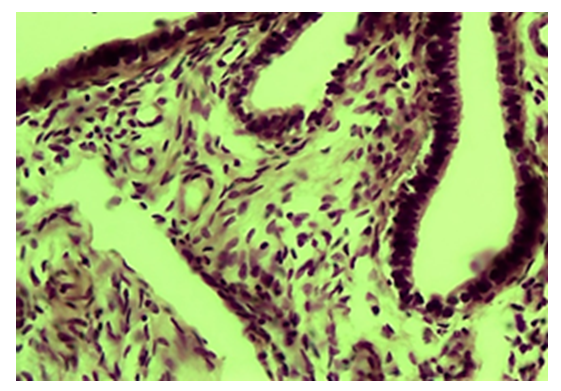 | Figure 2. Tubal-peritoneal infertility. The lamina propria contains many connective tissue cells and fibers. Color: G-E. UV: 10x40 |
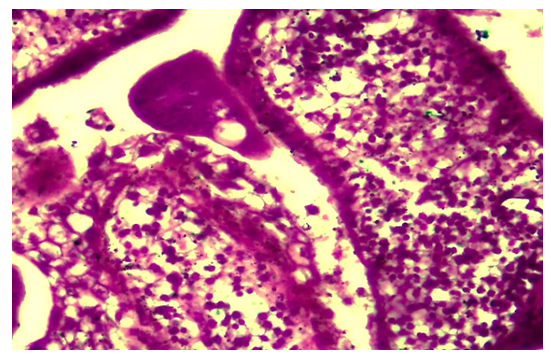 | Figure 3. Tubal-peritoneal infertility. The lamina propria of the villi of the tube mucosa is diffusely infiltrated with lymphoid cells. Color: G-E. UV: 10x40 |
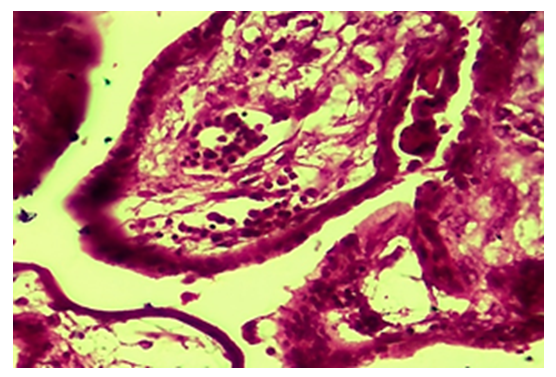 | Figure 4. Tubal-peritoneal infertility. Swelling, loosening and necrobiosis of the lamina propria of the villi of the mucous membrane of the uterine tube. Color: G-E. UV: 10x40 |
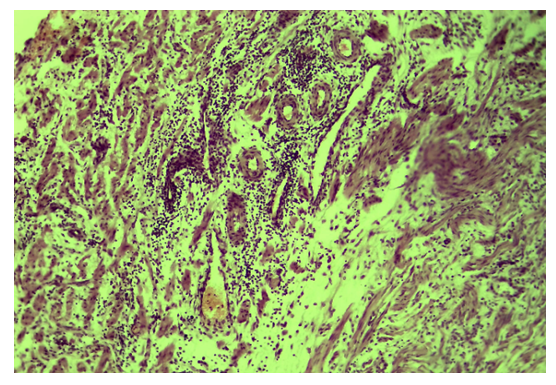 | Figure 5. Tubal-peritoneal infertility. Diffuse edema and inflammatory infiltration of the interstitium of all layers of the wall of the fallopian tube. Color: G-E. UV: 10x10 |
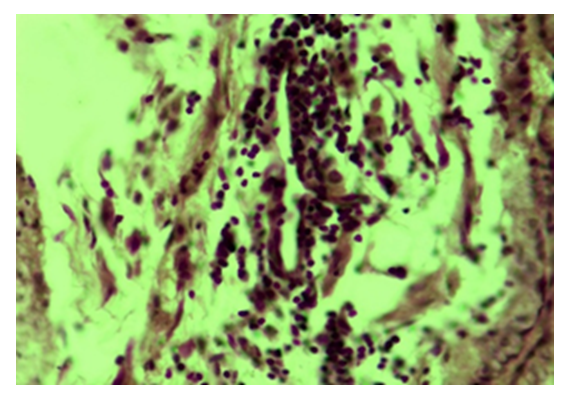 | Figure 6. Tubal-peritoneal infertility. Inflammatory infiltration of the wall of the vein vessel of the fallopian tube wall. Color: G-E. UV: 10x40 |
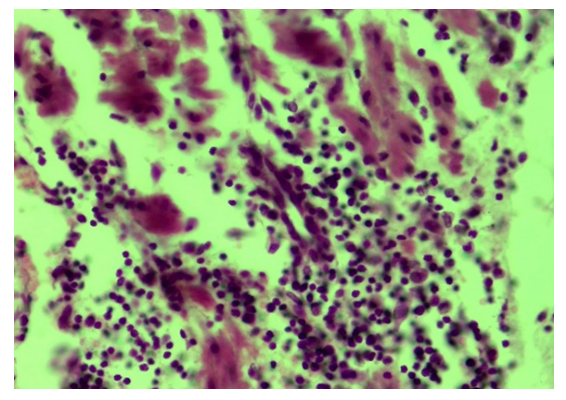 | Figure 7. Tubal-peritoneal infertility. Lymphoproliferative infiltration of the muscular layer of the fallopian tube wall. Oxarca: G-E. UV: 10x40 |
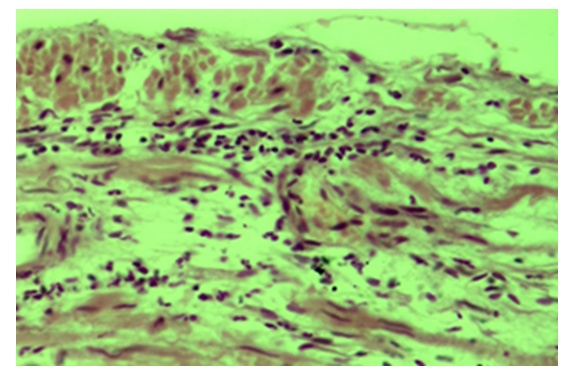 | Figure 8. Tubal-peritoneal infertility. The serous membrane of the tube wall is infiltrated with lymphohistiocytic cells. Color: G-E. UV: 10x10 |
4. Conclusions
- 1. It has been established that in tubal-peritoneal infertility, all layers of the uterine wall are subject to an inflammatory-sclerotic process.2. Discomplexation, deformation, inflammation and fibrosis of the lamina propria, atrophy, and metaplasia of the integumentary epithelium were noted.3. Diffuse lymphoproliferative inflammation of the submucosal and muscular layers with destruction of the tube’s own cell-fibrous structures was determined.4. Thickening of the serous membrane due to a set of edema and inflammatory infiltration, thickening of muscle cells with destruction and desquamation of the mesothelium.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML