| [1] | Kumar, S. & Singh, R. (2018). Pterygium excision and conjunctival autograft: a comparative study of techniques. Oman J Ophthalmol. 11(2): 124–128. |
| [2] | Akarsu C, Taner P, Ergin A. (2003). 5-Fluorouracil as chemoadjuvant for primary pterygium surgery: preliminary report. Cornea. 22(6): 522–526. |
| [3] | Al Fayez MF. (2002). Limbal versus conjunctival autograft transplantation for advanced and recurrent pterygium. Ophthalmology. 109(9): 1752–1755. |
| [4] | Allen CL, Clare G, Stewart EA, et al. (2013). Augmented dried versus cryopreserved amniotic membrane as an ocular surface dressing. PLoS One. 8(10): e78441. |
| [5] | Allen, B.D., Short, P. & Crawford, G.J. (1988). Pinguecula and pterygia. Surv Ophthalmol. 32:41–49. |
| [6] | Almond MC, Dastrup BT, Kaufman SC. (2012). 5-Fluorouracil and mitomycin C: adjuncts to pterygium surgery. In: Hovanesian JA, editor. Pterygium: Techniques and Technologies for Surgical Success. Thorofare, NJ: Slack Inc, 55–64. |
| [7] | Alpay A, Uğurbas SH, Erdogan B. (2009). Comparing techniques for pterygium surgery. Clin Ophthalmol. 3:69–74. |
| [8] | Alsmman AH, Radwan G, Abozaid MA, Mohammed UA, Abd Elhaleim NG. (2017). Preoperative subconjunctival combined injection of bevacizumab and mitomycin C before the surgical excision of primary pterygium: clinical and histological results. Clin Ophthalmol. 11: 493–501. |
| [9] | Altan-Yaycioglu R, Kucukerdonmez C, Karalezli A, Corak F, Akova YA. (2013). Astigmatic changes following pterygium removal: comparison of 5 different methods. Indian J Ophthalmol. 61(3): 104–108. |
| [10] | Amano S, Motoyama Y, Oshika T, Eguchi S, Eguchi K. (2000). Comparative study of intraoperative mitomycin C and beta irradiation in pterygium surgery. Br J Ophthalmol. 84(6): 618–621. |
| [11] | Anguria P, Kitinya J, Ntuli S, Carmichael T. (2014). The role of heredity in pterygium development. Int J Ophthalmol. 7(3): 563–573. |
| [12] | Arain MA, Yaqub MA, Ameen SS, Iqbal Z, Naqvi AH, Niazi MK. (2012). Amniotic membrane transplantation in primary pterygium compared with bare sclera technique. J Coll Physicians Surg Pak. 22(7): 440–443. |
| [13] | Arenas E, Garcia S. (2007). A scleral soft contact lens designed for the postoperative management of pterygium surgery. Eye Contact Lens. 33(1): 9–12. |
| [14] | Ari S, Caca I, Yildiz ZÖ, Sakalar YB, Dogan E.(2009). Comparison of mitomycin C and limbal-conjunctival autograft in the prevention of pterygial recurrence in Turkish patients: a one-year, randomized, assessor-masked, controlled trial. Curr Ther Res Clin Exp. 70(4): 274–281. |
| [15] | Aspiotis M, Tsanou E, Gorezis S, et al. (2007). Angiogenesis in pterygium: study of microvessel density, vascular endothelial growth factor, and thrombospondin-1. Eye. 21(8): 1095–1101. |
| [16] | Aydin A, Karadayi K, Aykan U, Can G, Colakoglu K, Bilge AH. (2008). Effectiveness of topical ciclosporin A treatment after excision of primary pterygium and limbal conjunctival autograft. J Fr Ophtalmol. 31(7): 699–704. |
| [17] | Balci M, Sahin S, Mutlu FM, Yağci R, Karanci P, Yildiz M. (2011). Investigation of oxidative stress in pterygium tissue. Mol Vis. 17: 443–447. |
| [18] | Bamdad S, Kooshki AS, Yasemi M. (2017). Surgical outcome of conjunctival rotational autograft-mitomycin C (MMC) versus free conjunctival autograft-MMC for pterygium removal: a randomized clinical trial. Electron Physician. 9(12): 5877–5884. |
| [19] | Barbados Eye Studies Group, Nemesure, B., Wu, S.Y., Hennis, A., & Leske, M.C. (2008). Nine-year incidence and risk factors for pterygium in the Barbados eye studies. Ophthalmology. 115: 2153–8. [PubMed: 18930552]. |
| [20] | Bekibele CO, Baiyeroju AM, Olusanya BA, Ashaye AO, Oluleye TS. (2008). Pterygium treatment using 5-FU as adjuvant treatment compared to conjunctiva autograft. Eye. 22(1): 31–34. |
| [21] | Besharati MR, Miratashi SA, Ahmadi AB. (2006). Pterygium surgery: amniotic membrane or conjunctival autograft transplantation. Int J Ophthalmol. 6: 1258–1262. |
| [22] | Bilge AD. (2018). Comparison of conjunctival autograft and conjunctival transposition flap techniques in primary pterygium surgery. Saudi J Ophthalmol.32(2):110–113. |
| [23] | Biswas MC, Shaw C, Mandal R. (2007). Treatment of pterygium with conjunctival limbal autograft and mitomycin C – a comparative study. J Indian Med Assoc. 105(4):200, 202, 204. |
| [24] | Cárdenas-Cantú E, Zavala J, Valenzuela J, Valdez-García JE. (2016). Molecular basis of pterygium development. Semin Ophthalmol. 2016, 31(6): 567–583. |
| [25] | Celik T. (2018). In situ blood coagulum versus sutures for autograft fixation after pterygium excision. Curr Eye Res. 43(8): 977–980. |
| [26] | Chen KH, Hsu WM. (2006). Intraoperative ethanol treatment as an adjuvant therapy of pterygium excision. Int J Biomed Sci. 2(4): 414–421. |
| [27] | Cho H, Chuck RS. (2012). Pterygium excision and placement of amniotic membrane grafts. In: Hovanesian JA, editor. Pterygium: Techniques and Technologies for Surgical Success. Thorofare, NJ: Slack Inc, 91–100. |
| [28] | Choudhury S, Dutta J, Mukhopadhyay S, et al. (2014). Comparison of autologous in situ blood coagulum versus sutures for conjunctival autografting after pterygium excision. Int Ophthalmol. 34(1):41–48. |
| [29] | Chowers I, Pe’er J, Zamir E, Livni N, Ilsar M, Frucht-Pery J. (2001). Proliferative activity and p53 expression in primary and recurrent pterygia. Ophthalmology. 108(5): 985–988. |
| [30] | Clearfield E, Hawkins BS, Kuo IC. (2017). Conjunctival autograft versus amniotic membrane transplantation for treatment of pterygium: findings from a Cochrane systematic review. Am J Ophthalmol. 182: 8–17. |
| [31] | Clearfield, E., Muthappan, V., Wang, X. & Kuo IC. (2016). Conjunctival autograft for pterygium. Cochrane Database Syst Rev., 2(2): CD011349. doi: 10.1002/14651858.CD011349.pub2. PMID: 26867004, PMCID: PMC5032146. |
| [32] | Cohen, R.A, McDonald, M.B. (1993). Fixation of conjunctival autografts with an organic tissue adhesive. Arch Ophthalmol., 111: 1167–1168. [PubMed: 8363455]. |
| [33] | Cornelius CR. (2017). Recurrence rate and complications of pterygium extended removal followed by extended conjunctival transplant. Cornea. 36(1): 101–103. |
| [34] | Coroneo MT. (1993). Pterygium as an early indicator of ultraviolet insolation: a hypothesis. Br J Ophthalmol. 77(11): 734–739. |
| [35] | Coroneo, M.T, Di Girolamo, N. & Wakefield, D. (1999). The pathogenesis of pterygia. Curr Opin Ophthalmol., 10: 282–8. [PubMed: 10621537]. |
| [36] | Dadeya S, Malik KP, Gulliani BP. (2002). Pterygium surgery: conjunctival rotation autograft versus conjunctival autograft. Ophthalmic Surg Lasers. 33: 269–274. |
| [37] | Daglioglu MC, Coskun M, Ilhan N, et al. (2014). The effects of soft contact lens use on cornea and patient’s recovery after autograft pterygium surgery. Cont Lens Anterior Eye. 37(3): 175–177. |
| [38] | Detels, R. & Dhir, S.P. (1967). Pterygium: A geographical study. Arch Ophthalmol. 78: 485–91. [PubMed: 6046844] |
| [39] | Detorakis ET, Drakonaki EE, Spandidos DA. (2000). Molecular genetic alterations and viral presence in ophthalmic pterygium. Int J Mol Med. 6(1): 35–41. |
| [40] | Di Girolamo N, Chui J, Coroneo MT, Wakefield D. (2004). Pathogenesis of pterygia: role of cytokines, growth factors, and matrix metalloproteinases. Prog Retin Eye Res. 23(2): 195–228. |
| [41] | Di Girolamo N, Kumar RK, Coroneo MT, Wakefield D. (2002). UVB-mediated induction of interleukin-6 and -8 in pterygia and cultured human pterygium epithelial cells. Invest Ophthalmol Vis Sci. 43(11): 3430–3437. |
| [42] | Di Girolamo N. (2012). Association of human papilloma virus with pterygia and ocular-surface squamous neoplasia. Eye. 26(2): 202–211. |
| [43] | Droutsas, K. & Sekundo, W. (2010). Epidemiology of pterygium. A Review. Ophthalmologe, 107, 511-2. |
| [44] | Duke-Elder SS. (1965). System of Ophthalmology. Vol. VIII. London: Henry Kimpton, 574. |
| [45] | Dushku N, John MK, Schultz GS, Reid TW. (2001). Pterygia pathogenesis: corneal invasion by matrix metalloproteinase expressing altered limbal epithelial basal cells. Arch Ophthalmol. 119(5): 695–706. |
| [46] | Ebrahimi ME, Kordi-Tamandani DM, Arish M. (2016). A novel approach to investigation of the pathogenesis of pterygium based on assessment of promoter hyper-methylation and expression profile of CTLA4 gene: a credible report of CTLA4 gene expression in human eye tissue. Gene. 583(2): 130–133. |
| [47] | Erogul, O., Erogul, L.E., Korkmaz, N.S., Celik, F., Dogan, M. & Gobeka, H.H. (2020). Histopathological Evaluation of Pterygium Patients with Type 2 Diabetes Mellitus. Beyoglu Eye J., 5(2): 122-128. doi: 10.14744/bej.2020.43434. PMID: 35098075, PMCID: PMC8784475. |
| [48] | Fernandes M, Sangwan VS, Bansal AK, et al. (2005). Outcome of pterygium surgery: analysis over 14 years. Eye. 19(11): 1182–1190. |
| [49] | Fonseca EC, Rocha EM, Arruda GV. (2018). Comparison among adjuvant treatments for primary pterygium: a network meta-analysis. Br J Ophthalmol. 102(6): 748–756. |
| [50] | Frucht-Pery J, Raiskup F, Ilsar M, Landau D, Orucov F, Solomon A. (2006). Conjunctival autografting combined with low-dose mitomycin C for prevention of primary pterygium recurrence. Am J Ophthalmol. 141(6): 1044–1050. |
| [51] | Fuest, M., Mehta, J.S. & Coroneo, M.T. (2017). New treatment options for pterygium. Expert Review of Ophthalmology, 12:3, 193-196, doi: https://doi.org/10.1080/17469899.2017.1324297. |
| [52] | Ghanavati SZ, Shousha MA, Betancurt C, Perez VL. (2014). Combined conjunctival autograft and overlay amniotic membrane transplantation, a novel surgical treatment for pterygium. J Ophthalmic Vis Res. 9(3): 399–403. |
| [53] | Gumus K, Erkilic K, Topaktas D, Colin J. (2011). Effect of pterygia on refractive indices, corneal topography, and ocular aberrations. Cornea. 30(1): 24–29. |
| [54] | Gumus K, Topaktas D, Göktaş A, Karakucuk S, Oner A, Mirza GE. (2012). The change in ocular higher-order aberrations after pterygium excision with conjunctival autograft: a 1-year prospective clinical trial. Cornea. 31(12): 1428–1431. |
| [55] | Ha SW, Park JH, Shin IH, Kim HK. (2015). Clinical analysis of risk factors contributing to recurrence of pterygium after excision and graft surgery. Int J Ophthalmol. 8(3): 522–527. |
| [56] | Hanahan D, Folkman J. (1996). Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 86(3): 353–364. |
| [57] | Hao Y, Ma DH, Hwang DG, Kim WS, Zhang F. (2000). Identification of antiangiogenic and antiinflammatory proteins in human amniotic membrane. Cornea. 19(3): 348–352. |
| [58] | Heindl, L.M. & Cursiefen, C. (2010). Pterygium: Etiology, clinical aspects and novel adjuvant therapies. Ophthalmologe, 107, 517-20. |
| [59] | Hilgers JH. (1960). Pterygium: its incidence, heredity and etiology. Am J Ophthalmol. 50(4): 635–644. |
| [60] | Hilmi MR, Che Azemin MZ, Mohd Kamal K, Mohd Tamrin MI, Abdul Gaffur N, Tengku Sembok TM. (2017). Prediction of changes in visual acuity and contrast sensitivity function by tissue redness after pterygium surgery. Curr Eye Res. 42(6): 852–856. |
| [61] | Hong HS, Lee J, Lee E, et al. (2009). A new role of substance P as an injury-inducible messenger for mobilization of CD29(+) stromal-like cells. Nat Med. 15(4): 425–435. |
| [62] | Hosal BM, Gürsel E. (2000). Mitomycin-C for prevention of recurrent pterygium. Ann Ophthalmol. 32(2): 107–109. |
| [63] | Hovanesian JA, Starr CE, Vroman DT, The ASCRS Cornea Clinical Committee. (2017). Surgical techniques and adjuvants for the management of primary and recurrent pterygia. J Cataract Refract Surg. 43(3): 405–419. |
| [64] | Ibáñez M, Eugarrios MF, Calderón DI. (2009). Topical cyclosporin A and mitomycin C injection as adjunctive therapy for prevention of primary pterygium recurrence. Ophthalmic Surg Lasers Imaging. 40(3): 239–244. |
| [65] | Jin J, Guan M, Sima J, et al. (2003). Decreased pigment epithelium-derived factor and increased vascular endothelial growth factor levels in pterygia. Cornea. 22(5): 473–477. |
| [66] | Jürgenliemk-Schulz IM, Hartman LJ, Roesink JM, et al. (2004). Prevention of pterygium recurrence by postoperative single-dose beta-irradiation: a prospective randomized clinical double-blind trial. Int J Radiat Oncol Biol Phys. 59(4): 1138–1147. |
| [67] | Kase S, Osaki M, Jin XH, et al. (2007). Increased expression of erythropoietin receptor in human pterygial tissues. Int J Mol Med. 20(5): 699–702. |
| [68] | Kase S, Takahashi S, Sato I, Nakanishi K, Yoshida K, Ohno S. (2007). Expression of p27(KIP1) and cyclin D1, and cell proliferation in human pterygium. Br J Ophthalmol. 91(7): 958–961. |
| [69] | Katırcıoglu YA, Altiparmak U, Engur Goktas S, Cakir B, Singar E, Ornek F. (2015). Comparison of two techniques for the treatment of recurrent pterygium: amniotic membrane vs conjunctival autograft combined with mitomycin C. Semin Ophthalmol. 30(5–6):321–327. |
| [70] | Kau HC, Tsai CC, Lee CF, et al. (2006). Increased oxidative DNA damage, 8-hydroxydeoxy-guanosine, in human pterygium. Eye. 2006, 20(7): 826–831. |
| [71] | Kaufman SC, Jacobs DS, Lee WB, Deng SX, Rosenblatt MI, Shtein RM. (2013). Options and adjuvants in surgery for pterygium: a report by the American Academy of Ophthalmology. Ophthalmology. 120(1): 201–208. |
| [72] | Keklikci U, Celik Y, Cakmak SS, Unlu MK, Bilek B. (2007). Conjunctival-limbal autograft, amniotic membrane transplantation, and intraoperative mitomycin C for primary pterygium. Ann Ophthalmol. 39(4): 296–301. |
| [73] | Kenyon, K.R., Wagoner, M.D., Hettinger, M.E. (1985). Conjunctival autograft transplantation for advanced and recurrent pterygium. Ophthalmology, 92: 1461–1470. [PubMed: 4080320]. |
| [74] | Khalfaoui T, Mkannez G, Colin D, et al. (2011). Immunohistochemical analysis of vascular endothelial growth factor (VEGF) and p53 expression in pterygium from Tunisian patients. Pathol Biol. 59(3): 137–141. |
| [75] | Kheirkhah A, Nazari R, Nikdel M, Ghassemi H, Hashemi H, Behrouz MJ. (2011). Postoperative conjunctival inflammation after pterygium surgery with amniotic membrane transplantation versus conjunctival autograft. Am J Ophthalmol. 152(5): 733–738. |
| [76] | Kheirkhah A, Safi H, Molaei S, Nazari R, Behrouz MJ, Raju VK. (2012). Effects of pterygium surgery on front and back corneal astigmatism. Can J Ophthalmol. 47(5):423–428. |
| [77] | Kim KW, Kim JC. (2018). Current approaches and future directions in the management of pterygium. Int J Ophthalmol. 1(5): 709–711. |
| [78] | Kim KW, Park SH, Kim JC. (2016). Fibroblast biology in pterygia. Exp Eye Res. 142: 32–39. |
| [79] | Kim KW, Park SH, Wee SW, Kim JC. (2013). Overexpression of angiogenin in pterygium body fibroblasts and its association with proliferative potency. Invest Ophthalmol Vis Sci. 54(9):6355–6362. |
| [80] | Kim, H.H., Mun, H.J., Park, Y.J, Lee, K.W & Shin, J.P. (2008). Conjunctivolimbal autograft using a Fibrin adhesive in pterygium surgery. Korean J Ophthalmol., 22: 147–54. [PMCID: PMC2629906] [PubMed: 18784440]. |
| [81] | Koç F, Demirbay P, Teke MY. (2002). Primer ve rekürren pterygiumda konjonktival otogreftleme. T Oft Gaz. 583–588. |
| [82] | Koranyi G, Artzén D, Seregard S, Kopp ED. (2012). Intraoperative mitomycin C versus autologous conjunctival autograft in surgery of primary pterygium with four-year follow-up. Acta Ophthalmol. 90(3):266–270. |
| [83] | Koranyi G, Seregard S, Kopp ED. (2004). Cut and paste: a no suture, small incision approach to pterygium surgery. Br J Ophthalmol. 88(7): 911–914. |
| [84] | Koranyi G, Seregard S, Kopp ED. (2005). The cut-and-paste method for primary pterygium surgery: long-term follow-up. Acta Ophthalmol Scand. 83(3):298–301. |
| [85] | Koranyi, G., Seregard, S. & Kopp, E.D. (2004). A no suture, small incision approach to pterygium surgery. Br J. Ophthalmol. 88:911–4. [PMCID: PMC1772242] [PubMed: 15205236] |
| [86] | Kria L, Ohira A, Amemiya T. (1996). Immunohistochemical localization of basic fibroblast growth factor, platelet derived growth factor, transforming growth factor-beta and tumor necrosis factor-alpha in the pterygium. Acta Histochem. 98(2): 195–201. |
| [87] | Küçükerdönmez C, Akova YA, Altinörs DD. (2007). Comparison of conjunctival autograft with amniotic membrane transplantation for pterygium surgery: surgical and cosmetic outcome. Cornea. 26(4): 407–413. |
| [88] | Kurian A, Reghunadhan I, Nair KG. (2015). Autologous blood versus fibrin glue for conjunctival autograft adherence in sutureless pterygium surgery: a randomised controlled trial. Br J Ophthalmol. 99(4):464–470. |
| [89] | Lan, A., Xiao, F., Wang, Y., Luo, Z. & Cao, Q. (2017). Efficacy of fibrin glue versus sutures for attaching conjunctival autografts in pterygium surgery: a systematic review with meta-analysis and trial sequential analysis of evidence. Oncotarget, 41487-41497. |
| [90] | Lee SB, Li DQ, Tan DT, Meller DC, Tseng SC. (2000). Suppression of TGF-beta signaling in both normal conjunctival fibroblasts and pterygial body fibroblasts by amniotic membrane. Curr Eye Res. 20(4): 325–334. |
| [91] | Lešin M, Paradžik M, Marin Lovrić J, et al. (2018). Cauterisation versus fibrin glue for conjunctival autografting in primary pterygium surgery (CAGE CUP): study protocol of a randomised controlled trial. BMJ Open. 8(6): e020714. |
| [92] | Lewallen, S. (1989). A randomized trial of conjunctival autografting for pterygium in the tropics. Ophthalmology, 96:1612–4. [PubMed: 2694049]. |
| [93] | Liang K, Jiang Z, Ding BQ, Cheng P, Huang DK, Tao LM. (2011). Expression of cell proliferation and apoptosis biomarkers in pterygia and normal conjunctiva. Mol Vis. 17: 1687–1693. |
| [94] | Lin H, Luo L, Ling S, et al. (2013). Lymphatic microvessel density as a predictive marker for the recurrence time of pterygium: a three-year follow-up study. Mol Vis. 19: 166–173. |
| [95] | Lindquist TP, Lee WB. (2015) Mitomycin C-associated scleral stromalysis after pterygium surgery. Cornea. 34(4): 398–401. |
| [96] | Ling S, Liang L, Lin H, Li W, Xu J. (2012). Increasing lymphatic microvessel density in primary pterygia. Arch Ophthalmol. 130(6):735–742. |
| [97] | Liu J, Fu Y, Xu Y, Tseng SC. (2012). New grading system to improve the surgical outcome of multirecurrent pterygia. Arch Ophthalmol. 130(1): 39–49. |
| [98] | Ma DH, See LC, Liau SB, Tsai RJ. (2000). Amniotic membrane graft for primary pterygium: comparison with conjunctival autograft and topical mitomycin C treatment. Br J Ophthalmol. 84(9): 973–978. |
| [99] | Mansour AM. (2017). Regression of inflamed pterygia by frequent high-dose intralesional ziv-aflibercept. Cornea. 36(8): 1002–1005. |
| [100] | Marchetti C, Sidahmed-Adrar N, Collin F, Jore D, Gardès-Albert M, Bonnefont-Rousselot D. (2011). Melatonin protects PLPC liposomes and LDL towards radical-induced oxidation. J Pineal Res. 51(3): 286–296. |
| [101] | Miah, M. R. (2012). A Framework of Global e-Learning for the Advanced Generations. Asian Business Review, 1(2), 145–151. https://doi.org/10.18034/abr.v1i2.135. |
| [102] | Miah, M. R. (2013). A Framework on Internet Banking Services for the Rationalized Generations. Asian Business Review, 3(2), 63–69. https://doi.org/10.18034/abr.v3i2.88. |
| [103] | Miah, M. R. (2018). Assessment of Environmental Policy Instruments along with Information Systems for Biodiversity Conservation in Bangladesh (Doctoral dissertation, PhD Thesis. IBEC, UNIMAS, Malaysia. Retrieved from https://ir.unimas.my/id/eprint/24535/. |
| [104] | Miah, M. R. (2020d). Concept of Cyber Antichrists, Chapter one. Cyber Dazzal: Sushaysther Ontoray (ed., pp. 1-128). Published by Paprhi Prakash, Sylhet, Bangladesh. Retrieved from https://www.rokomari.com/book/202988/cyber-dazzal---sushaysther-ontoray. |
| [105] | Miah, M. R., & Kar, N. (2017). MIS Practices in Banking Sector: A Case Study on Prime Bank Limited. Asian Accounting and Auditing Advancement, 8(1), 30–44. https://doi.org/10.18034/4ajournal.v8i1.47. |
| [106] | Miah, M. R., Hasan, M. M., Parisa, J. T., Alam, M. S. E., Shahriar, C. S., Akhtar, F., Begum, M., Sayok, A.K., Abdullah, F., Shamsuddin, M.A.S., Rahman, A.A.M.S., Alam, M.S., Tabassum, T., Chowdhury, S.H., Sharif, M.A., Rahman, M.S., Uddin, M.B., Tamim, M.A.K., Nazim, A.Y.M., Hannan, M.A., Uddin, M.J., Uddin, MB., Ghani, M.A., Nipa, N.S., Khan, M.S., Ahmed, G., Hossain, M.S., Rashid, M.M., Beg, M.O., Samdany, A.A., Hossain, S.A.M.I., Selim, M.A., Uddin, M.F., Nazrin, M.S., Azad, M.K.H., Malik, S.U.F., Hossain, M.K. & Chowdhury, M.A.K.. (2022b). Impact of Oscillated Wireless Sensor Networks to Initiate Cardiac Arrest. International Journal of Internal Medicine, 11(1), 1-17. url: http://article.sapub.org/10.5923.j.ijim.20221101.01.html, doi: https://doi.org/10.5923/j.ijim.20221101.01. |
| [107] | Miah, M. R., Hasan, M. M., Parisha, J. T., Chowdhury, S. H., Sayok, A. K., & Uddin, M. B. (2023). A Unique Revolutionary Journey across the Globe to Discover the Novel Coronavirus. International Journal of Research -GRANTHAALAYAH, 11(4), 84–100. https://doi.org/10.29121/granthaalayah.v11.i4.2023.5137. |
| [108] | Miah, M. R., Hasan, M. M., Parisha, J. T., Huda, M. B., Sher-E-Alam, M., Kiew Sayok, A., Rahman, M. S., Sharif, M. A., Uddin, M. B., Chowdhury, S. H., & Bhuiyan, M. A. (2023b). Misuse of Advanced Satellite Technology to Accelerate Man-made Flash Floods. International Journal of Research -GRANTHAALAYAH, 11(3), 160–171. https://doi.org/10.29121/granthaalayah.v11.i3.2023.5058. |
| [109] | Miah, M. R., Hasan, M. M., Parisha, J. T., Sayok, A. K., Uddin, M. B., Chowdhury, S. H. & Miah, M.M.U. (2023a). Impact of High Radio Frequency Satellite Oscillations on Initiating Earthquakes. International Journal of Research -GRANTHAALAYAH, 11(5), 129–197. https://doi.org/10.29121/granthaalayah.v11.i5.2023.5142. |
| [110] | Miah, M. R., Khan, M. S., Rahman, A. A. M. S., Samdany, A. A., Hannan, M. A., Chowdhury, S. H., & Sayok, A. K. (2020a). Impact of Sensor Networks towards Individuals Augmenting Causes of Diabetes. International Journal of Diabetes Research, 9(2), 1-10. doi: https://doi.org/10.5923/j.diabetes.20200902, url: http://article.sapub.org/10.5923.j.diabetes.20200902.02.html. |
| [111] | Miah, M. R., Mustaffa, M. S., Jayos, S., Ibrahim, N. H., Bujang, S., Saili, J., & Sayok, A. K. (2019). Towards Stimulating Tools for Advancement of Environmental Conservation through Promoting of Psychological Instruments. Journal of Sustainable Development, 12(4), 196-224. doi: https://doi.org/10.5539/jsd.v12n4p196. |
| [112] | Miah, M. R., Rahman, A. A. M. S., Khan, M. S., Hannan, M. A., Hossain, M. S., Shahriar, C. S., Hossain, S.A.M.I., Talukdar, M.T.H., Samdany, A.A., Alam, M.S., Uddin, M.B., Sayok, A.K. & Chowdhury, S. H. (2021a). Effect of Coronavirus Worldwide through Misusing of Wireless Sensor Networks. American Journal of Bioinformatics Research, 11(1), 1-31. url: http://article.sapub.org/10.5923.j.bioinformatics.20211101.01.html. doi: 10.5923/j.bioinformatics.20211101.01. |
| [113] | Miah, M. R., Rahman, A. A. M. S., Sayok, A. K., Samdany, A. A., & Hannan, M. A. (2021). How to fight the COVID-19 global crisis. World Journal of Environmental Research, 11(2), 31–38. https://doi.org/10.18844/wjer.v11i2.5855 |
| [114] | Miah, M. R., Sayok, A. K., Sarok, A., & Uddin, M. B. (2018a). Applications of Biological Diversity Information Systems towards Conservation at Lawachara National Park in Bangladesh. Malaysian Journal of Medical and Biological Research, 5(2), 93-104. https://doi.org/10.18034/mjmbr.v5i2.457. |
| [115] | Miah, M.M., Hasan, M.M., Parisha, J.T., Alam, M.S., Sayok, A.K., Rahman, M.S., Sharif, M.A., Uddin, M.B., & Chowdhury, S.H. (2023d). Innovative Policy Approach to Environmental Resource Management Through Green Banking Activities. American Journal of Economics, 13(2), 35-51. Retrieved from http://article.sapub.org/10.5923.j.economics.20231302.01.html. doi: 10.5923/j.economics.20231302.01. |
| [116] | Miah, M.R. & Shamsuddin, M.A.S. (2012). Impact of Environmental Education Technology in Secondary Educational Institutions: A Study in Chittagong City Area. Asian Journal of Applied Science and Engineering. 1(1), 44– 51. |
| [117] | Miah, M.R. & Shamsuddin, M.A.S. (2012a). A Framework of National Environmental Education: An Insight at Secondary Level Urban Schools. Journal of General Education, l (2), 1–10. |
| [118] | Miah, M.R. & Shamsuddin, M.A.S. (2013). Assessing of Teaching Learning through Environmental Education in Secondary Level: A Closer Outlook in Urban Areas. NAEM Journal, 8 (16). 05-12. |
| [119] | Miah, M.R. (2013). Enhancing Food Security through Acclimatized Species Domestication in the Haor Region. ABC Journal of Advanced Research, 2(1), 49–65. |
| [120] | Miah, M.R. (2020b). Cyber Dazzal: Barrier to good health (version Bangla language). Book on Contemporary Issues. Paprhi Prokash. 1-128. ISBN: 9789845860413. Retrieved from https://www.rokomari.com/book/202988/cyber-dazzal---sushaysther-ontoray. |
| [121] | Miah, M.R. (2020c). Corona virus wide-reaching through sensor technology, Chapter 18. Cyber Dajjal: Obstacle to good health. Paprhi Prakash, Rangmahal Tower, Bandar Bazar, Sylhet, Bangladesh, 115-121. ISBN: 978-984-586-041-3. url: https://rokomari.com/book/202988/cyber-dazzal---sushaysther-ontoray (Bengali language). |
| [122] | Miah, M.R. (2022j). Environmental Conservation Instruments: Dynamic Policy and Advanced Technology. LAMBERT Academic Publishing. 1-388. ISBN: 9786205490129. Retrieved from https://isbnsearch.org/isbn/9786205490129. https://www.amazon.co.uk/Environmental-Conservation-Instruments-Advanced-Technology/dp/6205490129. |
| [123] | Miah, M.R. (2023c). Discovery of Coronavirus (book). Scientific and Academic Publishing, California, USA. 1-345 [in press]. url: http://www.sapub.org/Book/index.aspx. |
| [124] | Miah, M.R., Alam, M.S., Hasan, M.M., Parisha, J.T., Sayok, A.K., Rahman, M.S., Sharif, M.A. & Uddin, M.B. (2022c). Scientific Environmental Governance to Accelerate Sustainable Biodiversity Management. Advances in Life Sciences, 11(1), 1-16. url: http://article.sapub.org/10.5923.j.als.20221101.01.html. doi: 10.5923/j.als.20221101.01. |
| [125] | Miah, M.R., Chowdhury, S.H., Parisha, J.T., Rashid, M.M., Hassan, M.M. & Sayok, A.K. (2023e). Impact of Radiofrequency Tracking on Body Surfaces for Acute Exacerbations of Skin Disease. American Journal of Dermatology and Venereology, 12 (1), 1-9. url: http://article.sapub.org/10.5923.j.ajdv.20231201.01.html, doi: 10.5923/j.ajdv.20231201.01. |
| [126] | Miah, M.R., Hannan, M.A., Rahman, AAMS., Khan, M.S., Hossain, M.M., Rahman, I.T., Hossain, M.S., Shahriar, C.S., Uddin, M.B., Talukdar, M.T.H., Alam, M.S., Hossain, S.A.M.I., Samdany, A.A., Chowdhury, S.H., Sayok, A.K. (2021b). Processed Radio Frequency towards Pancreas Enhancing the Deadly Diabetes Worldwide. Journal of Endocrinology Research, 3(1), 1-20. url: https://ojs.bilpublishing.com/index.php/jer/article/view/2826. doi: 10.30564/jer.v3i1.2826. |
| [127] | Miah, M.R., Hasan, M.M., Hannan, M.A., Parisa, J.T., Uddin, M.J., Uddin, M.B., Rahman, A.A.M.S., Hossain, S.A.M.I., Sharif, M.A., Akhtar, F., Shamsuddin, M.A.S., Alam, M.S.E., Alam, M.S., Abdullah, F., Rahman, M.S., Uddin, M.B., Shahriar, C.S., Sayok, A.K., Begum, M., Hossain, M.M., Khan, M.S., Ahmed, G., Malik, S.U.F., Samdany, A.A., Ghani, M.A., Hossain, M.S., Nazrin, M.S., Tamim, M.A.K., Selim, M.A., Talukdar, M.T.H., Chowdhury, F.T., Rashid, T.U., Nazim, A.Y.M., Rashid, M., Chowdhury, S.H. (2022). Myths about Coronavirus: A Research Defense. Global Journal of Health Science, 14(2), 63–112. url: https://ccsenet.org/journal/index.php/gjhs/article/view/0/46717. |
| [128] | Miah, M.R., Hasan, M.M., Miah, M.M.U., Parisha, J.T., Alam, M.S., Sayok, A.K., Rahman, M.S., Sharif, M.A. & Uddin, M.B. (2023f). Innovative Policy to Enable Sustained Conserving of Forest Biodiversity. International Journal of Agriculture and Forestry, 13(1), 1-22. url: http://article.sapub.org/10.5923.j.ijaf.20231301.01.html. doi: 10.5923/j.ijaf.20231301.01. |
| [129] | Miah, M.R., Hasan, M.M., Parisa, J.T., Alam, M.S., Akhtar, F., Begum, M., Shahriar, C.S., Sayok, A.K., Abdullah, F., Shamsuddin, M.A.S., Rahman, M.S., Sharif, M.A., Rahman, A.A.M.S., Alam, M.S., Uddin, M.B. and Chowdhury, S.H. (2021h). Unexpected Effects of Advanced Wireless Sensor Technology on Climate Change. World Environment, 11(2), 41-82. url: http://article.sapub.org/10.5923.j.env.20211102.01.html, doi: 10.5923/j.env.20211102.01. |
| [130] | Miah, M.R., Hasan, M.M., Parisha, J.T. & Chowdhury, S.H. (2022a). Socioeconomic Impact of the Coronavirus Pandemic with Multiple Factors on Global Healthcare Policy. Journal of Politics and Law, 15(4), 242. url: https://ccsenet.org/journal/index.php/jpl/article/view/0/47787. doi: https://doi.org/10.5539/jpl.v15n4p242. |
| [131] | Miah, M.R., Hasan, M.M., Parisha, J.T. & Sayok, A.K. (2022e). Challenges of Legal Instruments for Biodiversity Conservation along with National Parks. International Journal of Agriculture and Forestry, 12(3), 79-101. doi: 10.5923/j.ijaf.20221203.03. |
| [132] | Miah, M.R., Hasan, M.M., Parisha, J.T. & Sayok, A.K. (2023g). A Framework on Biodiversity Conservation Related Policy Analysis. American Journal of Environmental Engineering, 13(1), 1-12. url: http://article.sapub.org/10.5923.j.ajee.20231301.01.html., doi: 10.5923/j.ajee.20231301.01. |
| [133] | Miah, M.R., Hasan, M.M., Parisha, J.T. & Sayok, A.K. (2023h). A Framework on Biodiversity Conservation Related Policy Analysis. American Journal of Environmental Engineering, 13(1), 1-12. Retrieved from http://article.sapub.org/10.5923.j.ajee.20231301.01.html. doi: 10.5923/j.ajee.20231301.01. |
| [134] | Miah, M.R., Hasan, M.M., Parisha, J.T., Alam, M.S., Sayok, A.K., Rahman, M.S., Sharif, M.A., Uddin, M.B. & Chowdhury, S.H. (2022f). Impact of Processed Wireless Sensor Networks on Biodiversity Conservation. International Journal of Biological Engineering, 7(1), 1-13. Retrieved from http://article.sapub.org/10.5923.j.ijbe.20220701.01.html. doi: 10.5923/j.ijbe.20220701.01. |
| [135] | Miah, M.R., Hasan, M.M., Parisha, J.T., Alam, M.S., Sayok, A.K., Sarok, A. & Uddin, M.B. (2023i). Enhancing National Park Information Knowledge to Improve Biodiversity Conservation in Bangladesh: A Study on Policy Perspectives. International Journal of Plant Research, 13(1), 1-23. Retrieved http://article.sapub.org/10.5923.j.plant.20231301.01.html. doi: 10.5923/j.plant.20231301.01. |
| [136] | Miah, M.R., Hasan, M.M., Parisha, J.T., Chowdhury, S.H. & Sayok, A.K. (2023j). Misuse of Technology to Exacerbate Democracy in Crisis. American Journal of Sociological Research, 13(1), 12-23. url: http://article.sapub.org/10.5923.j.sociology.20231301.03.html. doi: 10.5923/j.sociology.20231301.03. |
| [137] | Miah, M.R., Hasan, M.M., Parisha, J.T., Chowdhury, S.H. & Sayok, A.K. (2023k). Misuse of Technology to Exacerbate Democracy in Crisis. American Journal of Sociological Research, 13(1), 12-23. Retrieved from http://article.sapub.org/10.5923.j.sociology.20231301.03.html. doi: 10.5923/j.sociology.20231301.03. |
| [138] | Miah, M.R., Hasan, M.M., Parisha, J.T., et al. Chowdhury, S.H. (2022g). Towards the Misuse of Advanced Wireless Sensor Technology to Enable the Sudden Onset of ARDS. American Journal of Medicine and Medical Sciences, 12(6), 616-638. doi: https://doi.org/10.5923/j.ajmms.20221206.05. |
| [139] | Miah, M.R., Hasan, M.M., Parisha, J.T., Sayok, A.K., Alam, M.S. & Chowdhury, S.H. (2022h). Issues and Challenges in Medical Jurisprudence Due to Misuse of Wireless Sensor Technology. American Journal of Medicine and Medical Sciences, 12(12), 1277-1291. url: http://article.sapub.org/10.5923.j.ajmms.20221212.23.html. doi: 10.5923/j.ajmms.20221212.23. |
| [140] | Miah, M.R., Hasan, M.M., Parisha, J.T., Sayok, A.K., Sarok, A., Uddin, M.B., Alam, M. S., Rahman, M.S., Miah, M.M.U., Sharif, M.A. & Hossain, M.A. (2023l). Biodiversity Information Systems in Geospatial Applications for Protected Area Management. American Journal of Geographic Information System, 12(1), 1-27. Retrieved from http://article.sapub.org/10.5923.j.ajgis.20231201.01.html. doi: 10.5923/j.ajgis.20231201.01. |
| [141] | Miah, M.R., Hasan, M.M., Parisha, J.T., Shahriar, C.S., Sayok, A.K., Chowdhury, S.H. (2022i). Adverse Global Health Impacts Due to the Proliferation of Man-Made Technological Heatwaves. Resources and Environment, 12(3), 67-75. url: http://article.sapub.org/10.5923.j.re.20221203.01.html, doi: 10.5923/j.re.20221203.01. |
| [142] | Miah, M.R., Hasan, M.M., Parisha, J.T., Shahriar, C.S., Sayok, A.K., Selim, M.A. & Chowdhury, S.H. (2023m). A Scientific Innovative Approach to Recovery from Dengue Fever. Public Health Research, 13(1), 1-14. url: http://article.sapub.org/10.5923.j.phr.20231301.01.html, doi: 10.5923/j.phr.20231301.01. |
| [143] | Miah, M.R., Hasan, MM., Parisa, J.T., Alam, MSE, Hossain, MM., Akhtar, F., Begum, M., Sayok, AK., Abdullah, F., Shamsuddin, MAS., Rahman, AAMS., Alam, MS., Chowdhury, SH. (2021g). Coronavirus: A Terrible Global Democracy. International Journal of Applied Sociology, 11(2), 46-82. url: http://article.sapub.org/10.5923.j.ijas.20211102.02.html, doi: 10.5923/j.ijas.20211102.02. |
| [144] | Miah, M.R., Mustaffa, M.S., Sabil, S., Madihie, A., Saili, J. & Sayok, A.K. (2018). Towards Dynamic Policy for Early Childhood Development Enhanced the Growth of Self-Regulations. International Journal of Engineering & Technology, 7(3.30), 251-255. Retrieved from https://www.sciencepubco.com/index.php/ijet/article/view/18251/8231. doi: https://doi.org/10.14419/ijet.v7i3.30.18251. |
| [145] | Miah, M.R., Rahman, A.A.M.S., Parisa, J.T., Hannan, M.A., Khan, M.S., Samdany, A.A., Sayok, A.K. and Chowdhury, S.H. (2021d). Discovery of Coronavirus with Innovative Technology. Science and Technology, 11(1), 7-29. url: http://article.sapub.org/10.5923.j.scit.20211101.02.html, doi: 10.5923/j.scit.20211101.02. |
| [146] | Miah, M.R., Rahman, A.A.M.S., Samdany, A.A., & Chowdhury, S.H. (2021e). A Dynamic Scientific Model for Recovery of Corona Disease. Frontiers in Science, 11(1), 1-17. url: http://article.sapub.org/10.5923.j.fs.20211101.01.html. doi: 10.5923/j.fs.20211101.01 |
| [147] | Miah, M.R., Rahman, AAMS., Hasan, M.M., Parisa, J.T., Hannan, M.A., Hossain, M.M., Alam, M.S., Alam, M.S.E., Akhtar, F., Ghani, M.A., Khan, M.S., Shahriar, C.S., Sayok, A.K., Begum, M., Malik, S.U.F., Samdany, A.A., Ahmed, G. and Chowdhury, S.H. (2021c). Adverse Effects of Wireless Sensor Technology to Debilitating in Numbness. International Journal of Virology and Molecular Biology, 10(1), 12-25. url: http://article.sapub.org/10.5923.j.ijvmb.20211001.03.html, doi: 10.5923/j.ijvmb.20211001.03. |
| [148] | Miah, M.R., Rahman, AAMS., Khan, M.S., Samdany, A.A., Hannan, M.A., Chowdhury, S.H., Sayok, A.K. (2020). Impact of Sensor Technology Enhancing Corona Disease. American Journal of Biomedical Engineering, 10 (1). 16–26. doi: 10.5923/j.ajbe.20201002. |
| [149] | Miah, M.R., Sayok, A.K., Rahman, AAMS, Samdany, A.A., Akhtar, F., Azad, A.K., Hasan, MM, Khan, M.S., Alam, S., Alam, MS., Uddin, M.B., Abdullah, F., Shahriar, C.S., Shamsuddin, MAS., Uddin, M.B., Sarok, A., Rahman, I.T., Chowdhury, S.C., Begum, M. (2021f). Impact of Sensor Networks on Aquatic Biodiversity in Wetland: An Innovative Approach, Geosciences, 11(1), 10-42. url: http://article.sapub.org/10.5923.j.geo.20211101.02.html, doi: 10.5923/j.geo.20211101.02. |
| [150] | Miah, M.R., Uddin, M.M., Parisha, J.T., Shahriar, C.S., Alam, M.S., Chowdhury, S.H., Nazim, A.Y.M., Hannan, M.A., Uddin, M.J., Uddin, M.B., Nipa, N.S., Khan, M.S., Ahmed, G., Hossain, M.S., Rashid, M.M., Samdany, A.A., Hossain, S.A.M.I., Selim, M.A., Uddin, M.F., Nazrin, M.S., Azad, MKH., Malik, SUF., Hossain, M.M., Chowdhury, M.A.K., Tanjil, Y., Talukdar, MTH., Rahman, AAMS., Sayok, A.K., Sharif, M., A., Rahman, MS., Hasan, M.M., Alam, M.S., Uddin, M.B., Patowary, D., Bhuiyan, MRA. & Chowdhury, MTR. (2023n). Uncontrolled Advanced Wireless Sensor Technology to Enable Early Growth of Stomach Cancer. American Journal of Stem Cell Research, 5(1), 8-39. url: http://article.sapub.org/10.5923.j.ajscr.20230501.02.html, doi: 10.5923/j.ajscr.20230501.02. |
| [151] | Mitra, S. (2011). Autoblood as Tissue Adhesive for Conjunctival Autograft Fixation in Pterygium Surgery, Poster Presented at the Annual Meeting of the American Academy of Ophthalmology, 22-23 October 2011, Orlando, Fla. |
| [152] | Mohammed I. (2011). Treatment of pterygium. Ann Afr Med. 10(3):197–203. |
| [153] | Moreno-López R. (2004). Estudio comparativo entre escisión de pterigión primario con autoinjerto conjuntival, membrana amniótica y cierre primario [Comparative study between primary pterygium excision using conjunctival autograft, amniotic membrane, and primary closure] Rev Mex Oftalmol. 78:291–297. |
| [154] | Mullins JB, Holds JB, Branham GH, Thomas JR. (1997). Complications of the transconjunctival approach. A review of 400 cases. Arch Otolaryngol Head Neck Surg. 123(4): 385–388. |
| [155] | Natung T, Keditsu A, Shullai W, Goswami PK, Sutureless GPK. (2017). Sutureless, glue-less conjunctival autograft versus conjunctival autograft with sutures for primary, advanced pterygia: an interventional pilot study. J Clin Diagn Res. 11(8): NC04–NC07. |
| [156] | Nepp, J., Abela, C., Polzer, I., Derbolav, A. & Wedrich, A. (2000). Is there a correlation between the severity of diabetic retinopathy and keratoconjunctivitis sicca? Cornea, 19: 487–91. |
| [157] | Nuzzi R, Tridico F. (2017). Efficacy of subconjunctival bevacizumab injections before and after surgical excision in preventing pterygium recurrence. J Ophthalmol. 2017(11): 6824670–6824677. |
| [158] | Nuzzi R, Tridico F. (2018). How to minimize pterygium recurrence rates: clinical perspectives. Clin Ophthalmol. 12: 2347-2362. doi: 10.2147/OPTH.S186543. PMID: 30538417, PMCID: PMC6251440. |
| [159] | Okabe M, Kitagawa K, Yoshida T, et al. (2014). Hyperdry human amniotic membrane is useful material for tissue engineering: physical, morphological properties, and safety as the new biological material. J Biomed Mater Res A. 102(3): 862–870. |
| [160] | Olusanya BA, Ogun OA, Bekibele CO, et al. (2014). Risk factors for pterygium recurrence after surgical excision with combined conjunctival autograft (CAG) and intraoperative antimetabolite use. Afr J Med Med Sci. 43(1): 35–40. |
| [161] | Ordman, L.J. & Gillman, T. (1996). Studies in the healing of cutaneous wound. Arch Surg. 93: 857–928. [PubMed: 954325]. |
| [162] | Ozcimen M, Sakarya Y, Goktas S, et al. (2015). Effect of nepafenac eye drops on pain associated with pterygium surgery. Eye Contact Lens. 41(3):187–189. |
| [163] | Özer A, Yıldırım N, Erol N, Yurdakul S. (2009). Long-term results of bare sclera, limbal-conjunctival autograft and amniotic membrane graft techniques in primary pterygium excisions. Ophthalmologica. 223(4): 269–273. |
| [164] | Ozgurhan EB, Kara N, Cankaya KI, et al. (2015). Corneal wavefront aberrations after primary and recurrent pterygium surgery. Eye Contact Lens. 41(6):378–381. |
| [165] | Pan X, Zhang D, Jia Z, Chen Z, Su Y. (2018). Comparison of hyperdry amniotic membrane transplantation and conjunctival autografting for primary pterygium. BMC Ophthalmol. 18(1):119. |
| [166] | Panda, A., Kumar, S., Kumar, A., Bansal, R. & Bhartiya, S. (2009). Fibrin glue in ophthalmology. Indian J Ophthalmol., 57: 371–9. [PMCID: PMC2804126] [PubMed: 19700876]. |
| [167] | Paracha Q, Ayoob M, Dawood Z, Mirza SA. (2014). Recurrence rate with use of intraoperative mitomycin C versus conjunctival autograft following pterygium excision. Pak J Med Sci. 30(6): 1243–1246. |
| [168] | Park CY, Choi JS, Lee SJ, Hwang SW, Kim EJ, Chuck RS. (2011). Cyclooxygenase-2-expressing macrophages in human pterygium co-express vascular endothelial growth factor. Mol Vis. 17: 3468–3480. |
| [169] | Peiretti E, Dessì S, Mulas C, et al. (2007). Modulation of cholesterol homeostasis by antiproliferative drugs in human pterygium fibroblasts. Invest Ophthalmol Vis Sci. 48(8): 3450–3458. |
| [170] | Pherwani A, Vakil V, Eatamadi H, Singh R, Dua HS. (2007). Postoperative subconjunctival 5-fluorouracil in the management of recurring pterygium. Br J Ophthalmol. 91(3): 398–399. |
| [171] | Pikkel J, Porges Y, Ophir A. (2001). Halting pterygium recurrence by postoperative 5-fluorouracil. Cornea. 20(2): 168–171. |
| [172] | Pinkerton OD, Hokama Y, Shigemura LA. (1984). Immunologic basis for the pathogenesis of pterygium. Am J Ophthalmol. 98(2):225–228. |
| [173] | Prabhasawat P, Tesavibul N, Leelapatranura K, Phonjan T. (2006). Efficacy of subconjunctival 5-fluorouracil and triamcinolone injection in impending recurrent pterygium. Ophthalmology. 113(7):1102–1109. |
| [174] | Prat D, Zloto O, Ben Artsi E, Ben Simon GJ. (2018). Therapeutic contact lenses vs tight bandage patching and pain following pterygium excision: a prospective randomized controlled study. Graefes Arch Clin Exp Ophthalmol. 256(11): 2143–2148. |
| [175] | Ratnalingam V, Eu AL, Ng GL, Taharin R, John E. (2010). Fibrin adhesive is better than sutures in pterygium surgery. Cornea. 29(5): 485–489. |
| [176] | Razeghinejad MR, Banifatemi M. (2014). Subconjunctival bevacizumab for primary pterygium excision, a randomized clinical trial. J Ophthalmic Vis Res. 9(1): 22–30. |
| [177] | Razmjoo H, Vaezi M-H, Peyman A, Koosha N, Mohammadi Z, Alavirad M. (2014). The effect of pterygium surgery on wavefront analysis. Adv Biomed Res. 3:196. |
| [178] | Reid TW, Dushku N. (2003). Does human papillomavirus cause pterygium? Br J Ophthalmol. 2003, 87(7): 806–808. |
| [179] | Ren Y, Wang C, Lin Y. (2009). Study on topical cyclosporine A in the prevention of pterygium recurrence. Int J Ophthalmol. 9:2240–2241. |
| [180] | Riau AK, Wong TT, Lan W, et al. (2011). Aberrant DNA methylation of matrix remodeling and cell adhesion related genes in pterygium. PLoS One. 6(2):e14687. |
| [181] | Rohrbach IM, Starc S, Knorr M. (1995). Vorhersage von Pterygiumrezidiven Aufgrund Morphologischer und Immunhistologischer Parameter [Predicting recurrent pterygium based on morphologic and immunohistologic parameters] Ophthalmologe. 92(4):463–468. |
| [182] | Rokohl, A.C. & Heindl, L.M. (2022). Pterygium: new insights into risk factors? Annals of Eye Science, 7(31). doi: https://dx.doi.org/10.21037/aes-22-30. |
| [183] | Rokohl, A.C., Heindl, L.M. & Cursiefen, C. (2021). Erratum to: Pterygium: pathogenesis, diagnosis and treatment. Ophthalmologe, 118, 179-80. |
| [184] | Rokohl, A.C., Heindl, L.M. & Cursiefen, C. (2021). Pterygium: pathogenesis, diagnosis and treatment. Ophthalmologe, 118, 749-63. |
| [185] | Romano V, Cruciani M, Conti L, Fontana L. (2016). Fibrin glue versus sutures for conjunctival autografting in primary pterygium surgery. Cochrane Database Syst Rev. 12(4): CD011308. |
| [186] | Rosen R. (2018). Amniotic membrane grafts to reduce pterygium recurrence. Cornea. 37(2):189–193. |
| [187] | Sánchez-Thorin, J.C., Rocha, G., Yelin, J.B. (1998). Meta-analysis on the recurrence rates after bare sclera resection with and without mitomycin C use and conjunctival autograft placement in surgery for primary pterygium. Br J Ophthalmol. 82:661–665. [PMCID: PMC1722618] [PubMed: 9797669]. |
| [188] | Sandra S, Zeljka J, Zeljka VA, Kristian S, Ivana A. (2014). The influence of pterygium morphology on fibrin glue conjunctival autografting pterygium surgery. Int Ophthalmol. 34(1):75–79. |
| [189] | Sangwan VS, Burman S, Tejwani S, Mahesh SP, Murthy R. (2007). Amniotic membrane transplantation: a review of current indications in the management of ophthalmic disorders. Indian J Ophthalmol. 55(4): 251–260. |
| [190] | Sarnicola, V., Vannozzi, L. & Motolese, P.A. (2010). Recurrence rate using fibrin glue-assisted ipsilateral conjunctival autograft in pterygium surgery: 2-year follow-up. Cornea, 29:1211–4. [PubMed: 20697275]. |
| [191] | Segev F, Jaeger-Roshu S, Gefen-Carmi N, Assia EI. (2003). Combined mitomycin C application and free flap conjunctival autograft in pterygium surgery. Cornea. 22(7): 598–603. |
| [192] | Shahin MM, Elbendary AM, Elwan MM, Maha M, Amal M, Mohamed M. (2012). Intraoperative subconjunctival bevacizumab as an adjunctive treatment in primary pterygium: a preliminary report. Ophthalmic Surg Lasers Imaging. 43(6): 459–466. |
| [193] | Sharma A, Gupta A, Ram J, Gupta A. (2000). Low-dose intraoperative mitomycin-C versus conjunctival autograft in primary pterygium surgery: long term follow-up. Ophthalmic Surg Lasers. 31(4): 301–307. |
| [194] | Sharma, A.K., Wali, V. & Pandita, A. (2004). Corneo conjunctival auto grafting in pterygium surgery. J Med Educ Res., 6:149–52. |
| [195] | Shenasi A, Mousavi F, Shoa-Ahari S, Rahimi-Ardabili B, Fouladi RF. (2011). Subconjunctival bevacizumab immediately after excision of primary pterygium: the first clinical trial. Cornea. 30(11): 1219–1222. |
| [196] | Shera, A.S., Jawad, F., Maqsood, A., Jamal, S., Azfar, M., Ahmed, U. (2004). Prevalence of chronic complications and associated factors in type 2 diabetes. J. Pak. Med. Assoc. 54:54–9. |
| [197] | Shimazaki J, Kosaka K, Shimmura S, Tsubota K. (2003). Amniotic membrane transplantation with conjunctival autograft for recurrent pterygium. Ophthalmology. 110(1): 119–124. |
| [198] | Shusko, A., Schechter, B.A. & Hovanesian, J.A. (2020). Pterygium Surgery Utilizing Limbal Conjunctival Autograft and Subconjunctival Amniotic Membrane Graft in High-Risk Populations. Clin Ophthalmol., 14:2087-2090. doi: 10.2147/OPTH.S243584. PMID: 32801617, PMCID: PMC7399472. |
| [199] | Siak JJK, Ng SL, Seet LF, Beuerman RW, Tong L. (2011). The nuclear-factor κB pathway is activated in pterygium. Invest Ophthalmol Vis Sci. 52(1):230–236. |
| [200] | Sihota R, Tondon R. [editors]. (2003). Parson's Diseases of Eye. 19th ed. India: Butterworth-Heinemann, 2003. Diseases of conjunctiva, 193-4. |
| [201] | Solomon A, Grueterich M, Li DQ, Meller D, Lee SB, Tseng SC. (2003). Overexpression of insulin-like growth factor-binding protein-2 in pterygium body fibroblasts. Invest Ophthalmol Vis Sci. 44(2): 573–580. |
| [202] | Solomon A, Pires RT, Tseng SC. (2001). Amniotic membrane transplantation after extensive removal of primary and recurrent pterygia. Ophthalmology. 108(3): 449–460. |
| [203] | Song YS, Ryu YH, Choi SR, Kim JC. (2005). The involvement of adult stem cells originated from bone marrow in the pathogenesis of pterygia. Yonsei Med J. 46(5): 687–692. |
| [204] | Spaeth, E.B. (1926). Rotational island graft for pterygium. Am J Ophthalmol., 9:649–55. |
| [205] | Stival LR, Lago AM, Figueiredo MN, Bittar RH, Machado ML, Nassaralla Junior JJ. (2014). Efficacy and safety of subconjunctival bevacizumab for recurrent pterygium. Arq Bras Oftalmol. 77(1):4–7. |
| [206] | Sul S, Korkmaz S, Alacamli G, Ozyol P, Ozyol E. (2018). Application of autologous serum eye drops after pterygium surgery: a prospective study. Graefes Arch Clin Exp Ophthalmol. 256(10):1939–1943. |
| [207] | Sun Y, Zhang B, Jia X, Ling S, Deng J. (2018). Efficacy and safety of bevacizumab in the treatment of pterygium: an updated meta-analysis of randomized controlled trials. J Ophthalmol. 2018:4598173–4598179. |
| [208] | Suzuki, T., Sano, Y. & Kinoshita, S. (2000). Conjunctival inflammation induces Langerhans cell migration into the cornea. Curr Eye Res., 21:550–3. [PubMed: 11035535]. |
| [209] | Syam PP, Eleftheriadis H, Liu CS. (2003). Inferior conjunctival autograft for primary pterygia. Ophthalmology. 110(4): 806–810. |
| [210] | Tan, D.T, Chee, S.P, Dear, K.B, Lim, A.S. (1997). Effect of pterygium morphology on pterygium recurrence in a controlled trial comparing conjunctival autografting with bare sclera excision. Arch Ophthalmol., 115: 1235–1240. [PubMed: 9338666]. |
| [211] | Tananuvat N, Martin T. (2004). The results of amniotic membrane transplantation for primary pterygium compared with conjunctival autograft. Cornea. 23(5):458–463. |
| [212] | Tang B, Ren H, Liu H, et al. (2016). CCR5 blockade combined with cyclosporine A attenuates liver GVHD by impairing T cells function. Inflamm Res. 65(11):917–924. |
| [213] | Teng CC, Patel NN, Jacobson L. (2009). Effect of subconjunctival bevacizumab on primary pterygium. Cornea. 28(4): 468–470. |
| [214] | Thatte S. (2011). Amniotic membrane transplantation: an option for ocular surface disorders. Oman J Ophthalmol. 4(2): 67–72. |
| [215] | Ti, S.E, Chee, S.P., Dear, K.B. & Tan, D.T. (2000). Analysis of variation in success rates in conjunctival autografting for primary and recurrent pterygium. Br J Ophthalmol., 84:385–9. [PMCID: PMC1723439] [PubMed: 10729295]. |
| [216] | Tsai YY, Chiang CC, Yeh KT, Lee H, Cheng YW. (2010). Effect of TIMP-1 and MMP in pterygium invasion. Invest Ophthalmol Vis Sci. 2010, 51(7):3462–3467. |
| [217] | Uy, H.S., Reyes, J.M., Flores, J.D. & Lim-Bon-Siong, R. (2005). Comparison of fibrin glue and sutures for attaching conjunctival autografts after pterygium excision. Ophthalmology, 112: 667–671. [PubMed: 15808260]. |
| [218] | Valezi VG, Schellini SA, Hata Viveiros MM, Padovani CR. (2009). Segurança e efetividade no tratamento do pterígio usando infiltração de 5-fluoruracila no intraoperatorio [Safety and efficacy of intraoperative 5-fluorouracil infiltration in pterygium treatment] Arq Bras Oftalmol. 72:169–173. Portuguese. |
| [219] | Varssano D, Shalev H, Lazar M, Fischer N. (2013). Pterygium excision with conjunctival autograft: true survival rate statistics. Cornea. 32(9):1243–1250. |
| [220] | Viani GA, Stefano EJ, de Fendi LI, Fonseca EC. (2008). Long-term results and prognostic factors of fractionated strontium-90 eye applicator for pterygium. Int J Radiat Oncol Biol Phys. 72(4): 1174–1179. |
| [221] | Vichare, N., Choudhary, T., Arora, P. (2013). A comparison between fibrin sealant and sutures for attaching conjunctival autograft after pterygium excision. Med J Armed Forces India, 69: 151–5. [PMCID: PMC3862656] [PubMed: 24600089]. |
| [222] | Vrabec MP, Weisenthal RW, Elsing SH. (1993). Subconjunctival fibrosis after conjunctival autograft. Cornea. 1993, 12(2): 181–183. |
| [223] | Wang IJ, Lai WT, Liou SW, et al. (2000). Impression cytology of pterygium. J Ocul Pharmacol Ther. 2000, 16(6): 519–528. |
| [224] | Wu WK, Wong VW, Chi SC, Lam DS. (2007). Surgical management of double-head pterygium by using a novel technique: conjunctival rotational autograft combined with conjunctival autograft. Cornea. 26(9):1056–1059. |
| [225] | Yalcin Tok O, Burcu Nurozler A, Ergun G, Akbas Kocaoglu F, Duman S. (2008). Topical cyclosporine A in the prevention of pterygium recurrence. Ophthalmologica. 222(6): 391–396. |
| [226] | Ye J, Kook KH, Yao K. (2006). Temporary amniotic membrane patch for the treatment of primary pterygium: mechanisms of reducing the recurrence rate. Graefes Arch Clin Exp Ophthalmol. 244(5): 583–588. |
| [227] | Ye J, Song YS, Kang SH, Yao K, Kim JC. (2004). Involvement of bone marrow-derived stem and progenitor cells in the pathogenesis of pterygium. Eye. 18(8):839–843. |
| [228] | Yeung SN, Lichtinger A, Kim P, et al. (2015). Efficacy and safety of patching vs bandage lens on postoperative pain following pterygium surgery. Eye. 29(2):295–296. |
| [229] | Young AL, Tam PM, Leung GY, Cheng LL, Lam PT, Lam DS. (2009). Prospective study on the safety and efficacy of combined conjunctival rotational autograft with intraoperative 0.02% mitomycin C in primary pterygium excision. Cornea. 28(2):166–169. |
| [230] | Yüksel, B., Unsal, S.K. & Onat, S. (2010). Comparison of Fibrin glue and suture technique in pterygium surgery performed with limbal autograft. Int J Ophthalmol., 3:316–20. [PMCID: PMC3340741] [PubMed: 22553582]. |
| [231] | Zeng W, Liu Z, Dai H, et al. (2017). Anti-fibrotic, anti-VEGF or radiotherapy treatments as adjuvants for pterygium excision: a systematic review and network meta-analysis. BMC Ophthalmol. 17(1): 211. |
| [232] | Zhang Q, Bao N, Liang K, Tao L. (2018). Adjuvant use of cyclosporine a in the treatment of primary pterygium: a systematic review and meta-analysis. Cornea. 37(8): 1000–1007. |
| [233] | Zhang Z, Yang Z, Pan Q, Chen P, Guo L. (2018). Clinicopathologic characteristics and the surgical outcome of conjunctival granulomas after pterygium surgery. Cornea. 37(8): 1008–1012. |
| [234] | Zloto O, Rosen N, Leshno A, Rosner M. (2017). Very long-term success of pterygium surgery with conjunctival graft. Cont Lens Anterior Eye. 40(4): 267–269. |
| [235] | Reza, M.S., Miah, M.R., Chowdhury, F.T. & Chowdhury, M.A.K. (2023). Common Disease Profiles of Outpatients in Ophthalmology Department of Tertiary Care Hospital. International Journal of Optics and Applications, 9(1), 22-30. doi: 10.5923/j.optics.20230901.02. |
| [236] | Chowdhury, M.A.K., Reza, M.S., Miah, M.R., Jahangir, S.M., Chowdhury, M.T.T. & Ahmed, J.U. (2023). Effects of Pharmacological Agents on Glaucoma in Daily Life. International Journal of Optics and Applications, 9(1), 1-21. doi: 10.5923/j.optics.20230901.01. |
| [237] | Chowdhury, S.H., Rashid, M., Miah, M.R., Shahriar, C.S. & Tabassum, T. (2021). Effect of Skin Diseases in Modernized Life. American Journal of Dermatology and Venereology, 10(2), 13-24. doi: 10.5923/j.ajdv.20211002.01. Retrieved from http://article.sapub.org/10.5923.j.ajdv.20211002.01.html. |
| [238] | Miah, M. R., Sayok, A., Sarok, A., & Uddin, M. B. (2017a). Rain Water Harvesting for Sustainable Biodiversity Conservation at Lawachara National Park in Bangladesh: A Study on Policy Challenges. OIDA International Journal of Sustainable Development, 10 (01), 11-26. Available at SSRN: https://ssrn.com/abstract=2911866. |
| [239] | Ghoz, N., Elalfy, M., Said, D. & Dua, H. (2018). Healing of autologous conjunctival grafts in pterygium surgery. Acta Ophthalmologica, e979-e988. |
| [240] | WHO. (2022). Local-level policy recommendations: operationalizing a One Health approach – Political statement of the WHO European Healthy Cities Network. Annual Business meeting and technical conference 2022. World Health Organization- European Region. WHO/EURO: 2023-7060-46826-68259. |
| [241] | Salman, A.G. & Mansour, D.E. (2011). The recurrence of pterygium after different modalities of surgical treatment. Saudi J Ophthalmol. 25(4): 411-5. doi: 10.1016/j.sjopt.2010.10.013. Epub 2010 Oct 25. PMID: 23960956, PMCID: PMC3729301. |
| [242] | Parisha, J.T., Miah, M.R., Hasan, M.M., & Begum, M. (2022). Impact of Environmental Pollution along with Technology for Conserving of Biodiversity. International Journal of Ecosystem, 12(1), 20-30. url: http://article.sapub.org/10.5923.j.ije.20221201.02.html, doi: 10.5923/j.ije.20221201.02. |
| [243] | Miah, M. R., Sayok, A. K., Sarok, A., & Uddin, M. B. (2017). Towards Dynamic Policy Instruments for Enhancing Biodiversity Conservation in National Parks: A Case Study on Bangladesh and Sarawak, Malaysia. Borneo Journal of Resource Science and Technology, 7(1), 11-30. doi: https://doi.org/10.33736/bjrst.391.2017. |
| [244] | Miah, M.R., Hossain, M.M., Akhter, J. & Pasha, M.K. (2006). Diversity in Timber and Fruit Plants in the Nurseries of Chittagong Metropolitan Area. Chittagong University Journal of Biological Sciences, 30(1), 1-10. |


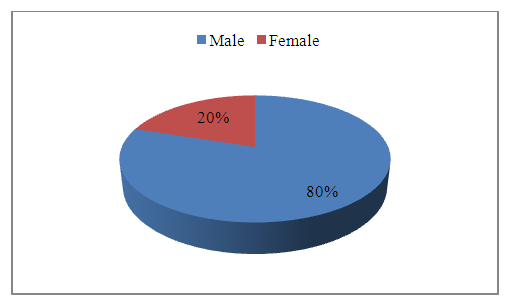
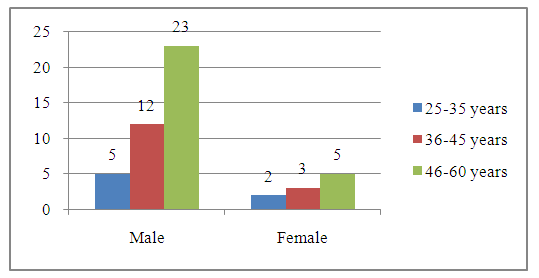
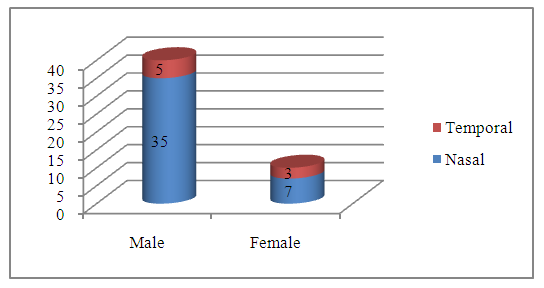
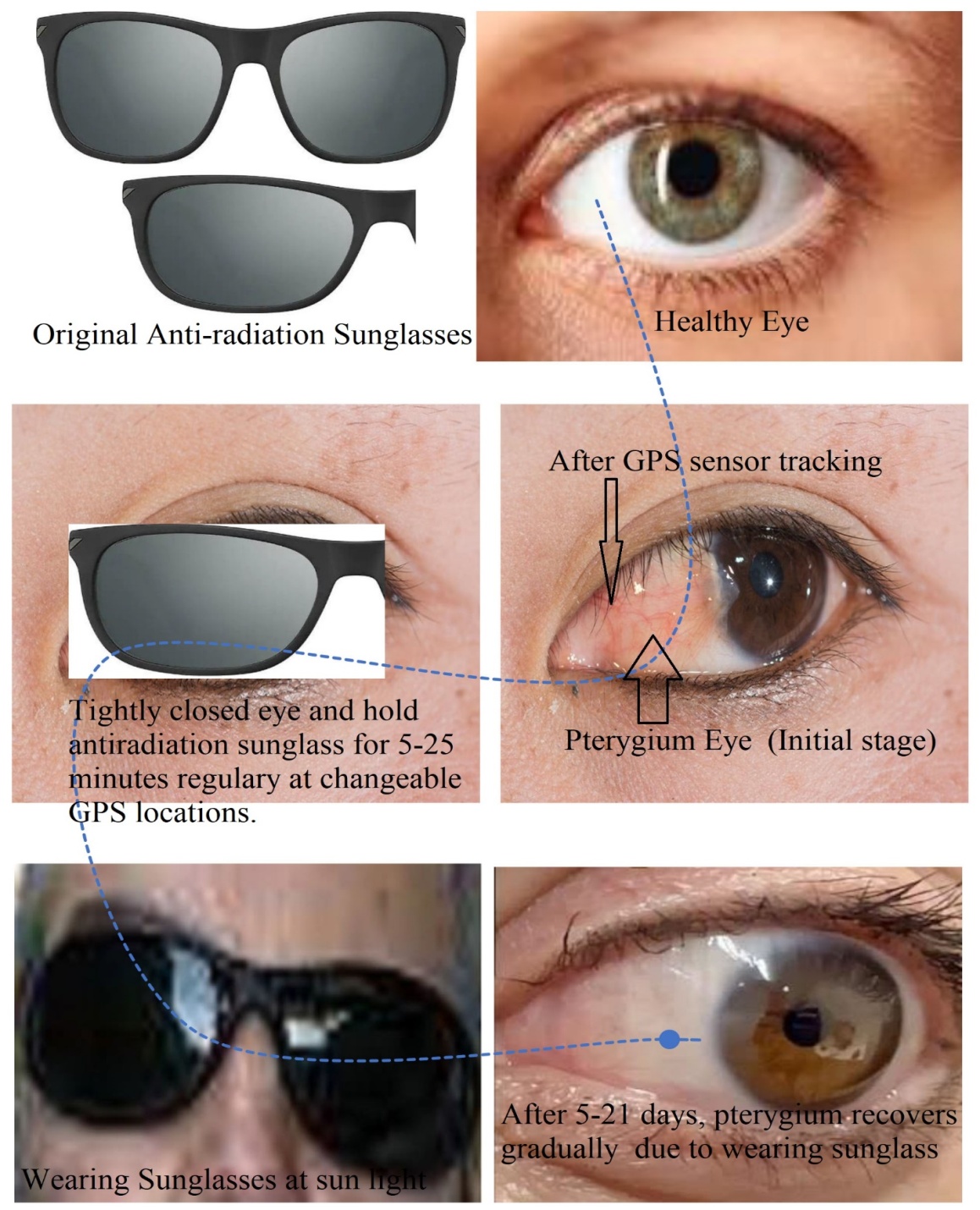
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML