-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2023; 13(11): 1735-1738
doi:10.5923/j.ajmms.20231311.31
Received: Oct. 18, 2023; Accepted: Nov. 12, 2023; Published: Nov. 21, 2023

Immunological Assessment of Changes in Various Organs and Tissues in Students with Observed Intestinal Dysbiosis
Ergashov Ozodzhon Ilkhomovich
Basical doctorant (PhD) of Tashkent Medical Academy, Tashkent City, Uzbekistan
Correspondence to: Ergashov Ozodzhon Ilkhomovich, Basical doctorant (PhD) of Tashkent Medical Academy, Tashkent City, Uzbekistan.
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

149 young students who were considered practically healthy were selected for the study. Bacteriological analysis of the intestinal microbiota of students, 48% of whom had dysbiosis of 1-2 degrees. When studying the quantitative indicators of specifically sensitive antigen-binding lymphocytes (ABL) to various tissue antigens (TAg) in the blood of these students, there was pre-pathology and pathology in the tissues, and it was found that pathological changes were 1.5 times (125/84) higher than changes in the organs and tissues of students with normal microflora.
Keywords: Student youth, Intestinal microbiocenosis, Antigen-binding lymphocytes, Tissue antigens
Cite this paper: Ergashov Ozodzhon Ilkhomovich, Immunological Assessment of Changes in Various Organs and Tissues in Students with Observed Intestinal Dysbiosis, American Journal of Medicine and Medical Sciences, Vol. 13 No. 11, 2023, pp. 1735-1738. doi: 10.5923/j.ajmms.20231311.31.
Article Outline
1. Introduction
- According to the World Health Organization (WHO), approximately 95.0% of the world's population suffers from intestinal dysbiosis. The intestinal microbiota (IM) is a separate element of the human body and has not been studied enough. The intestinal microbiota performs physiological and biochemical functions that seriously regulate the "host" organism, and are not considered as a set of symbiotic living bacteria only in the intestine [7,9]. Modern studies show that mi has protective, metabolic, trophic and immunological functions and is able to create "reciprocal relationships" that include cellular and soluble elements of mucosal immunity [6]. L.P. According to Knyshova and co-authors, the ability of the intestinal microbiota to detoxify can be compared with the ability of the liver. Modern studies show that disorders of the intestinal microbial-tissue complex are primary not only in the formation of liver steatosis, but also in the formation of pancreatic pathologies [3]. IM interactions in immune system interactions include homeostasis and inflammation in the intestine and, in some cases, in extra-intestinal tissues. Translational microbiome therapy can be used to treat chronic diseases by balancing the relationship between the macroorganism and microflora [12]. T- and B-lymphocytes make up the bulk of antigen-binding lymphocytes (ABL). Due to the pathological process in the body, the higher the level of cell destruction and necrosis in organs, the higher the level of sensitized ABL in the blood compared to tag [8,10]. In immunological screening, the use of ABL is an extremely effective method, in particular, the experiments of Yu. A. Kuzmin showed that ABL can be detected in the blood 12 hours after the antigenic stimulus, i.e. long before the formation of antibodies [4].Stress observed in adolescence, diet, alcohol consumption, and irregular use of antibiotics lead to changes in the gut microbiota [14]. The preservation of health, its management in the interests of life achievements is an urgent problem of national importance for the protection of youth health, although it is a personal matter for each of us, but as the main labor potential of society in the near future [2,11,13].
2. The Purpose of the Study
- The method of immunological screening of changes in organs and tissues of students with observed intestinal dysbiosis in the analysis of the health status of students.
3. Material and Methods of Research
- The research work was carried out in the problem laboratory "Clinical Microbiology, Mycology and Immunology" at the Department of Microbiology, Virology and Immunology of the Tashkent Medical Academy. Our study involved 149 "practically healthy" young people aged 18 to 25 years by their consent. Students received material for bacteriological (1 g of feces) and immunological (5 ml of peripheral venous blood) tests. The average age of students is 21.0±0.2 years. Based on a special survey, data were collected on the absence of acute or chronic diseases of the digestive system in the student's anamnesis, information about the nutrition of students, mild complaints characteristic of intestinal microflora disorders, and the fact that Ham had not taken antibiotics for the last 1 month. Based on the collected data from practically healthy students, material for bacteriological taxa was obtained. Sampling and seeding it into the food medium was carried out on the basis of Order 177 MHO and on the basis of other regulatory documents [5]. All the studied biological samples were planted by the Gold method in bloody agar, monitol-solitol-agar, Saburo and Endo mucous media, and it was found that the concentration of more than 103 CFU/ml was significant. The isolated cultures were identified according to their morphological, tinctorial, cultural and biochemical characteristics.For an immunological study, 5 ml of micdor blood was taken from the ulnar vein in the morning on an empty stomach and placed in test tubes with an isotonic solution consisting of 2.0 ml of sodium chloride and 2-3 drops of heparin. Lymphocytes from heparinized blood, 2 ml of ficolla–verografin gradient (density d= 1,077 g/ml) were poured into test tubes and centrifuged at a speed of 1500 ro/m. the district attorney was suspended for 30 minutes. Quantitative indicators of antigen-binding lymphocytes (ABL) in relation to tissue antigens in the blood (brain-control, liver, lungs, endocardium, myocardium, kidneys, endometrium, pancreas, prostate gland) F.Yu. were studied by the method of Garib and co-authors by indirect rosette formation (IRF) reaction [1]. The reaction results were visualized under an immersion microscope. The normal ABL index of 1.5±0.7% compared to the tissue antigen, the range of 4.4± 1.4% of the acquired pathological condition, 8.4±2.8% was estimated as a predisposition to the development of pathological processes.
4. Results and Their Analysis
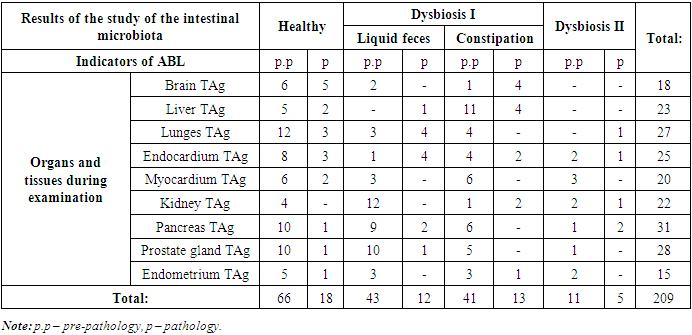
- In the IM analysis, out of 149 students who participated in the study, students diagnosed with dysbiosis accounted for 72 (48.3%), students with an ABL frequency higher than 1.5±0.7% in immunological analysis accounted for 101 (67.8%), and absolutely healthy students in both analyses accounted for 34 (22.8%). IM tests were normal, the pathology obtained by ABL indicators and pathological indicators observed by students were observed in 43 patients (28.8%), of which the obtained pathology indicators were observed in 28 students (18.8%), pathological indicators were observed in 15 students (10.0%).According to the dysbiosis index (DI) proposed by Sh.K. Adilov and co-authors, a total of 72 students (48.3%) were found to have I-II levels of dysbiosis. Of these, 63 (42.3%) received a grade of DI from students and 9 (6%) received a grade of DI II from students. The results of the immunological taxil of 72 students who had dysbacteriosis showed the frequency of ABL with pathology (25.5%) and pathological indicators (13.4%) in 58 of them (38.9%). In 14 students (9.4%) who were diagnosed with dysbacteriosis, the frequency of ABL in immunological analyses in the studied tissues was normal.During the immunological examination of students diagnosed with dysbiosis of the I degree, ABL received pathology in one and several tissues - 23.5% of the occurrence in pathogens, 2.0% of the occurrence in only 1 tissue according to pathological indicators, received pathology in several tissues, and the joint occurrence of pathological changes was observed in 9.4% of the number of students. Taking into account the presence of constipation in 23 students (15.4%) according to the results of a special survey conducted before laboratory tests among students who had dysbiosis I, we decided to highlight changes in the organs and tissues of these students in Table 1 separately.
|
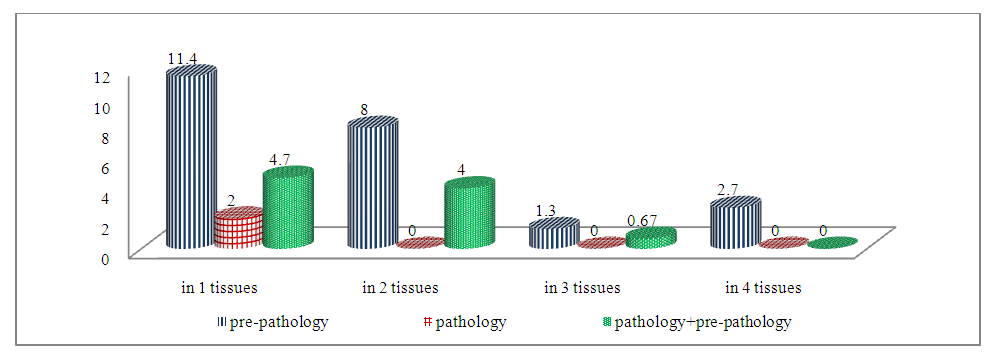 | Figure 1. Indicators of progression of grade I dysbiosis by TAg opposite ABL in one or more members and tissues of the observed students |
|
5. Conclusions
- 1. According to the results of bacteriological analysis, the degree of organ and tissue damage of students who were diagnosed with dysbiosis was 80.5% (58/72), and in students who did not have dysbiosis, the degree of organ and tissue damage was 55.8% (43/77). Also, in all students who were diagnosed with dysbiosis, the ABL level in 2 or more people exceeded the norm.2. In one or more of the 101 students who participated in the study, a total of 209 cases showed an increase in the tag counter ABL index from the norm, while injuries in the extremities (125) on the background of dysbiosis were 1.5 times higher than injuries in the extremities (84) on the background of normal microflora.3. Pre-pathology of dysbiosis and pathology against the background of changes in levels I - II were found mainly in 16 cases in the pancreas and prostate gland of the prostate gland, in 15 - in the kidneys, in 12 - in the myocardium, in 11 - in the liver, as well as pathological changes in the endocardium, in 7 - in the liver and lungs and 4 in the pancreas.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML