-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2023; 13(11): 1715-1720
doi:10.5923/j.ajmms.20231311.27
Received: Oct. 31, 2023; Accepted: Nov. 16, 2023; Published: Nov. 18, 2023

Features of Certain Hemostasis and Vascular Endothelium Parameters in Perinatal Nervous System Injuries in Newborns
Ziyadullaeva Khulkar Oblakulovna, Dilmuradova Klara Ravshanovna
Samarkand State Medical University, Samarkand City, Amir-Temur Street 18, Uzbekistan
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The pathogenesis of perinatal nervous system injuries in newborns is fundamentally linked to perinatal hypoxia. In this study, the authors conducted research on the state of the hemostasis system, vascular endothelium, and ultrasound examinations of the central nervous system in newborns with perinatal nervous system injuries. The findings revealed a statistically significant increase in the levels of fibrinogen and endothelin-1 in umbilical cord blood among newborns with perinatal nervous system injuries. Hypoxic injuries to the nervous system in newborns primarily trigger responses from vascular endothelium, leading to the activation of hemostasis disorders and cerebral blood flow alterations.
Keywords: Hypoxia, Asphyxia, Depth of the anterior horn of the lateral ventricles, Intraventricular hemorrhage, Cerebral edema, Endothelin-1, Fibrinogen
Cite this paper: Ziyadullaeva Khulkar Oblakulovna, Dilmuradova Klara Ravshanovna, Features of Certain Hemostasis and Vascular Endothelium Parameters in Perinatal Nervous System Injuries in Newborns, American Journal of Medicine and Medical Sciences, Vol. 13 No. 11, 2023, pp. 1715-1720. doi: 10.5923/j.ajmms.20231311.27.
Article Outline
1. Introduction
- Perinatal hypoxia holds one of the leading positions among perinatal factors that exert influence not only on the fetal condition but also on the characteristics of the neonatal period, ultimately impacting the health and subsequent development of the child [20,21]. The frequency of perinatal hypoxia shows no tendency to decrease. Approximately 60-80% of all central nervous system (CNS) disorders in childhood are associated with perinatal hypoxia [2]. According to literature data, about half of the children recognized as disabled are affected by cerebral palsy (CP), the formation of which in the majority of cases has a perinatal hypoxic-ischemic origin [18]. In the structure of childhood disability, the frequency of CNS (central nervous system) disorders accounts for 50%, of which 40% are cases of children with disabilities due to perinatal CNS injuries [19].More than half of all cases of CNS dysfunction in young children are attributed not to acute hypoxia during childbirth but to prolonged and chronic hypoxia during fetal and neonatal stages [3,12].Perinatal CNS injuries are characterized by their widespread occurrence and severe disability among affected individuals. According to Ratner A.Yu (2001), 7-19% of newborns show signs of nervous system damage, with a high neonatal mortality rate (40-50%) resulting from perinatal brain injuries, as reported by Badalyan L.O (1990). Perinatal stroke, as reported by Wu Y (2006), occurs at a frequency of 1 in 4000 newborns. Perinatal nervous system injuries lead to disability in 15-30% of full-term newborns and 40-60% of preterm newborns [4].From available literature data, it is known that 73% of all CNS injuries are due to various forms of hypoxia, 13% are related to CNS developmental abnormalities, 7% are caused by mechanical birth injuries, and 7% are associated with infectious diseases affecting the brain membranes [5].Despite the advances in perinatal medicine, the reduction in the frequency of CNS injuries in newborns remains relatively insignificant, which became the subject of our research.Among perinatal brain injuries, cerebrovascular pathology occupies a prominent position. Disturbances in cerebral hemodynamics play a crucial role in the development of hemorrhagic and ischemic brain injuries [16].In cases of ischemic brain injuries, cerebral angiodystonia with increased arterial tone and the development of intracranial hypertension more frequently formed. On the other hand, hemorrhagic brain injuries were more characterized by vasodilation, venous dystonia, and post-hemorrhagic hydrocephalus [9].Hypoxia and acidosis lead to significant changes in hemodynamics, neurodynamics, and cerebrospinal fluid dynamics, contributing to the occurrence of cerebral hypoperfusion [17].Insufficient oxygen supply leading to the development of hypoxic-ischemic encephalopathy (HIE) can occur through two mechanisms: hypoxemia, which restricts oxygen access to the blood, and ischemia, which reduces cerebral blood flow. Both mechanisms are associated with asphyxia in the form of hypoxia. As a result of anaerobic glucose metabolism, each molecule of glucose yields only two molecules of ATP compared to 38 molecules under aerobic conditions. The lack of oxygen in brain cells leads to a decrease in the amount of phosphocreatine and ATP. A reduction in ATP by more than 30% can occur within 6 minutes. Glycolysis accelerates, but impaired blood flow hinders glucose uptake, leading to the depletion of high-energy phosphates and the accumulation of lactate [13,14].The mechanisms of cell damage and necrosis during hypoxia and ischemia are not merely a result of energy deficiency. They are secondary initiating factors of a cascade of destructive events when the critical level of energy deficit is reached. Excessive membrane depolarization and the release of excitatory neurotransmitters, primarily glutamate, lead to massive calcium influx through NMDA and AMPA membrane receptors. Calcium activates various lipases, proteases, and nucleases, resulting in the breakdown of essential cellular proteins [10].The pathogenesis of hypoxic brain injury can be divided into two stages:1. The ischemic phase, predominantly involving necrotic processes in ischemic areas. Brain cell necrosis occurs during the acute injury, characterized by brain cell swelling, membrane destruction, leakage of intracellular substances, and immune system activation, leading to neuroinflammation.2. The reperfusion phase, occurring 2 to 6 hours after the experienced hypoxia, is characterized by a prevalence of apoptosis in cells and determines the therapeutic window during which the majority of changes are reversible. In this phase, processes initiating a cascade of pathophysiological events predominate: calcium influx and free radical formation, accumulation of free iron, and increased NO2- synthesis, culminating in cell apoptosis [8].There are three main groups of causes leading to the development of chronic intrauterine hypoxia, which can progress into neonatal hypoxia:1. Extragenital maternal diseases - smoking, neurocirculatory dystonia, thyroid gland disorders, obesity, diabetes, bronchial asthma, anemia, and heart rhythm disorders.2. Impaired uteroplacental circulation - hypertension or arterial hypotension in the mother.3. Impaired fetal-placental circulation - placental abruption, umbilical cord entanglement around the neck or body, true knots in the umbilical cord [2].During complicated labor (rapid or cesarean section), there is a reduction in placental circulation, leading to fetal hypoxia and acute asphyxia [15]. Hypoxia is recognized as the primary etiological factor of perinatal nervous system pathology, cerebrovascular disorders, and the development of hemorrhagic and ischemic CNS injuries in newborns [16].The topography of hypoxic brain tissue damage depends on the architecture of the vascular bed. In premature infants, the most vulnerable areas with the fewest capillaries and anastomoses are the periventricular regions, which are severely affected by hypoxia and result in the destruction of periventricular brain tissue, known as periventricular leukomalacia (PL). Basal ganglia and thalamus are also affected. The localization and type of brain tissue damage also depend on the duration of hypoxia. Acute asphyxia mainly leads to focal lesions in the form of thrombosis, resulting in regional necrosis, while chronic oxygen deprivation of nervous tissue is accompanied by diffuse changes, such as cytotoxic edema, which is more characteristic of premature infants [1,11,17].У newborns with hypoxic injuries to the nervous system, an increase in endothelin-1 (ET-1) in umbilical cord blood was observed in all groups. Under the influence of hypoxia, transcription of informational RNA is activated, leading to the synthesis of ET precursors, their conversion into ET-1, and its secretion within a few minutes [7].The main mechanism of action of ET-1 involves the activation of calcium release, leading to:1. Stimulation of platelet adhesion and aggregation and secondary hemostasis.2. Contraction and growth of smooth muscle cells in blood vessels, leading to vessel wall thickening and vasoconstriction [7].The significant increase in ET-1 levels in the blood, accompanied by a marked reduction in endothelial nitric oxide production, indicates a prevalence of vasoconstriction over vasodilation in patients who experienced acute cerebral blood flow disturbances. This prevalence leads to the occurrence of vasospasm and decreased blood flow. The degree of brain tissue damage correlates directly with the severity of endothelial dysfunction. ET-1 affects cerebral autoregulation processes by narrowing cerebral blood vessels and reducing cerebral blood flow below the ischemic threshold, which can trigger cerebral infarction. It has been found that ET-1 initiates the spasm of cerebral arteries through direct action on the vascular wall and by inducing neuronal depolarization due to the activation of endothelin type A receptors and phospholipase C [7].
2. Objectives
- The aim of this study was to investigate certain parameters related to the hemostasis system, vascular endothelium, as well as the clinical manifestations and neurosonography features in newborns with perinatal nervous system injuries.
3. Materials and Methods
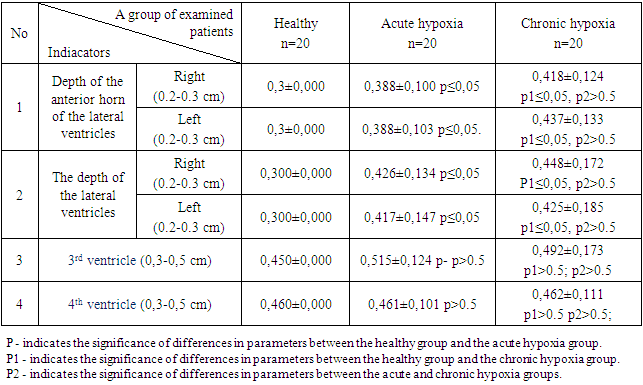
- The study included 60 newborn infants of different gestational ages, who were observed at the Department of Physiology and Neonatal Intensive Care of the Samarkand Regional Perinatal Center.The inclusion criteria for the study groups were as follows:Group I: Comprised 20 healthy newborns born to healthy mothers aged 21 to 33 years, with a normal obstetric history, physiological course of pregnancy, and delivery. Among them, 12 were full-term infants, and 8 were "conditionally healthy" preterm infants. The "conditionally healthy" preterm infants were born between 35 to 37 weeks of gestation, with a body weight ranging from 1500 to 2500 grams.Group II: Consisted of 20 infants who experienced acute asphyxia during childbirth, born to healthy mothers. The causes of acute hypoxia were cesarean section (5), nuchal cord (5), prolonged labor (7), and footling or breech presentation (3). The clinical presentation was characterized by a syndrome of increased neuro-reflex excitability, which included regurgitation, sleep disturbances, chin tremor, restlessness, spontaneous Moro reflex (Phase I), and a syndrome of depression characterized by muscle hypotonia, hypodynamia, weak sucking, horizontal nystagmus, and gastrointestinal dyskinesia.Group III: Consisted of 20 newborns who experienced chronic intrauterine hypoxia.The inclusion criteria for children in this group were as follows:1. Oligohydramnios (reduced amniotic fluid).2. Disturbances in fetal motor activity.3. Lag in the height of the uterine fundus from the gestational age norm and the absence of an increase in the uterine fundus height in dynamics.4. Changes in fetal heart rate. In normal conditions, fetal heart rate should be in the range of 110-170 beats per minute. Acceleration (more than 170 beats per minute) and deceleration (less than 110 beats per minute) of fetal heart rate.5. Coloration of the amniotic fluid. This is caused by the presence of meconium, which is released from the fetal intestines in response to oxygen deprivation.6. Doppler assessment of blood flow in the umbilical artery allows for an indirect evaluation of fetal hemodynamics.7. Cardiotocography: Changes in fetal heart rate, such as acceleration or deceleration.These criteria were used to identify and include newborns with chronic intrauterine hypoxia in the study group.The chronic intrauterine hypoxia was caused by the following factors: severe anemia (5 cases), exacerbation of chronic pyelonephritis (5 cases), severe preeclampsia (4 cases), threatened abortion and vomiting during pregnancy (4 cases), prolonged gestosis (1 case), and complete placenta previa (1 case). The infants in this group had low Apgar scores of 1-3 points, a complicated obstetric and gynecological history, and more pronounced signs of immaturity.At birth, the condition of these infants was severe or extremely severe, with suppression of physiological reflexes, requiring prolonged respiratory support. They were cared for in the neonatal intensive care unit and received intensive therapy. Neurological examination of these infants revealed the absence of reactions to stimuli and pain, adynamia, areflexia, atonia, weak or absent pupillary response to light, and sometimes localized eye symptoms. Their skin appeared cyanotic, pale with a "marbled shade" (indicating microcirculation disorder). They had shallow breathing with intercostal retractions. Cardiac sounds were muffled, and palpation of the abdomen revealed moderate hepatomegaly.Laboratory investigations included:1. Coagulogram: Prothrombin time (PT), Prothrombin index according to Quick (PI), International normalized ratio (INR), activated partial thromboplastin time (APTT), fibrinogen, and thrombin time (TT) were determined using the Human clot junior (2000) apparatus.2. Specific marker of endothelial dysfunction, endothelin-1 in the blood, was determined using the enzyme-linked immunosorbent assay on the Mindray VS-380 apparatus.Ultrasound examination of the brain structure using B-mode (neurosonography) was conducted on the GE Logic F8 apparatus (USA) with the use of multi-frequency convex probes of 5.5 MHz.The diagnosis of perinatal encephalopathies, depending on the nervous system impairments, was established in accordance with the classification of perinatal nervous system injuries in newborns by Sarnat and Sarnat (1976).The statistical analysis of the collected data was performed using specialized software, specifically Statistica 10.0 and Microsoft Excel (version 29, IDV Co., Armonk, NY, USA).
4. Results and Discussion
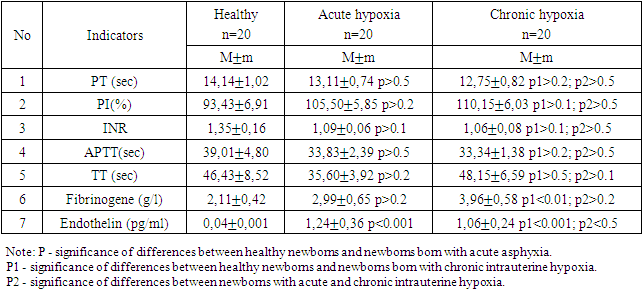
- During the investigation of hemostasis parameters, such as PT (prothrombin time), INR (international normalized ratio), APTT (activated partial thromboplastin time), and TT (thrombin time), in cases of acute and chronic neonatal hypoxia, no statistically significant differences were observed. The PT in acute asphyxia was 13.11±0.74 seconds, and in chronic hypoxia, it was 12.75±0.82 seconds on average. The corresponding values for the prothrombin index were 105.50±5.85 seconds and 110.15±6.03 seconds in acute and chronic hypoxia, respectively. Significant reductions in INR and APTT levels were noted in the studied neonates compared to those in healthy newborns.It is important to mention that no statistically significant differences were found in PT, TT, PI, INR, and APTT values between the patients and healthy newborns.However, among the hemostasis parameters analyzed in umbilical cord blood, only the fibrinogen level showed a statistically significant difference between healthy newborns and those who experienced chronic intrauterine hypoxia, with a difference of up to 3.96±0.58 g/L (p<0.01). No statistically significant difference in fibrinogen levels was observed between acute asphyxia and chronic hypoxia (p>0.2).The hemostasis and vascular endothelium parameters in newborns at one day of life are presented in the table 1 below (M±SD).
|
|
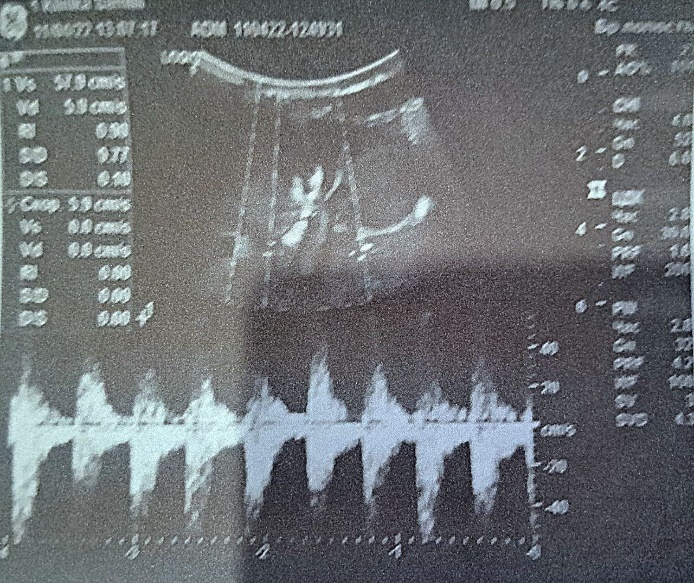 | Figure 1. Hypoxic changes in the periventricular region and basal ganglia |
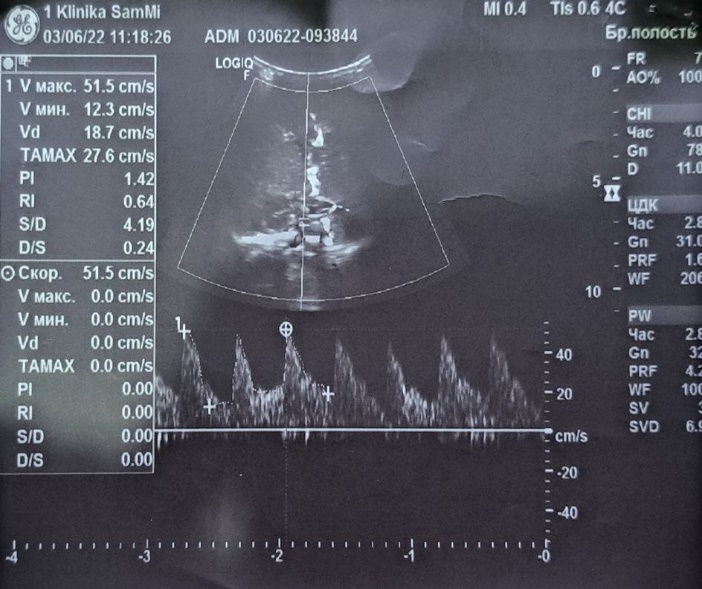 | Figure 2. Grade 1 intraventricular hemorrhage (IVH) |
5. Conclusions
- The conducted study has revealed that in cases of hypoxic brain injuries in newborns, the vascular endothelium reacts primarily, leading to the activation of disturbances in hemostasis and cerebral blood flow.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML