-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2023; 13(11): 1685-1688
doi:10.5923/j.ajmms.20231311.20
Received: Oct. 3, 2023; Accepted: Nov. 6, 2023; Published: Nov. 10, 2023

Morphological Characteristics of Congenital Heart Disease Tetralogy of Fallot in Bukhara Region
Jumaev A. U.1, Eshbaev E. A.2, Allaberganov D. SH.2
1Bukhara State Medical Institute, Bukhara, Uzbekistan
2Tashkent Medical Academy, Tashkent, Uzbekistan
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The regional distribution of congenital heart defects in Uzbekistan varies; morphological aspects of Fallot's tetralogy, which accounts for 8.7% of congenital heart defects in Bukhara province, have been studied. Histioarchitectonics, which is one of the characteristic aspects of congenital heart disease or blue-type heart defect, has been studied in Bukhara region. In this study, Fallot's tetralogy was morphologically characterised by the finding of hypertrophic and dystrophic changes in the right ventricular myocardium, atrophic and sclerotic changes in the left ventricular cardiomyocytes.
Keywords: Morphology, Congenital heart disease, Myocardial tetralogy of Fallot, Dextroposition, Atrophy, Hypertrophy, Sclerosis
Cite this paper: Jumaev A. U., Eshbaev E. A., Allaberganov D. SH., Morphological Characteristics of Congenital Heart Disease Tetralogy of Fallot in Bukhara Region, American Journal of Medicine and Medical Sciences, Vol. 13 No. 11, 2023, pp. 1685-1688. doi: 10.5923/j.ajmms.20231311.20.
1. Introduction
- The congenital heart defect identified by the French physician Etienne Louis Arthur Fallot is one of the most complex malformations among all congenital heart defects. Fallot's tetralogy is manifested in the heart by pulmonary artery stenosis, interchamber barrier defect, aortic disposition and right ventricular hypertrophy. These pathological changes are manifested by specific histotopographic changes in the cardiac myocardium. Based on this, the modern classification of congenital heart disease has been proposed in many different variants of nomenclature. These classifications are explained by the frequency of regional occurrence, incomplete compliance, origin of congenital heart defects, genetic, environmental, occupational factors. Congenital heart defects account for 20.8 per cent of all heart diseases in Uzbekistan. Every 100 children in Uzbekistan have a congenital heart defect, so 0.08 per cent of them are Fallot's tetralogy and are treated only surgically. To be more precise, if 530 thousand children are born in Uzbekistan on average per year, 1% of them (5300) have congenital heart defects, 25% of them are patients who need surgery, and 8% of 5300 are Fallot's tetralogy. enough. This numerically means that 106 children with the diagnosis of Fallot's tetralogy are born in Uzbekistan every year.The relevance of the problem is to study the distribution of Fallot's tetralogy in Bukhara region of the Republic of Uzbekistan and its peculiarities from the morphological point of view. In Bukhara oblast, the average number of diagnosed congenital heart defects per year is 32-38 Fallot's tetralogy.The aim: It consists in studying and analysing the level of occurrence of congenital heart defects in Bukhara region, anatomical, histological and morphometric changes.
2. Material and Methods
- In the Expert Bureau of Pathological Anatomy of the Bukhara region, clinical and anamnestic data of heart tissue and medical history obtained at autopsy of 65 children who died of congenital heart disease were analysed. Sections of heart tissue taken by morphological method were frozen in 10% buffered formalin for 72 hours. Then after washing in wastewater for 1 hour, they are dehydrated in ascending alcohols (70,80,90,100%). The slices are then frozen in paraffin and poured into cassettes. Using a microtome, 5-7 µm thick slices are made, deparaffinised in xylene and stained with haematoxylin and eosin. The obtained results are viewed under a light microscope, microphotographs are taken and morphometrically analysed.
3. Results and Discussion
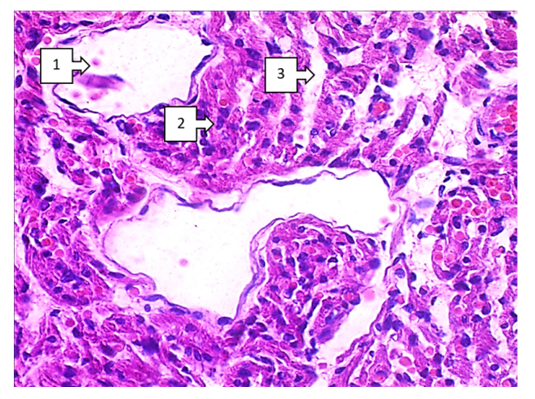
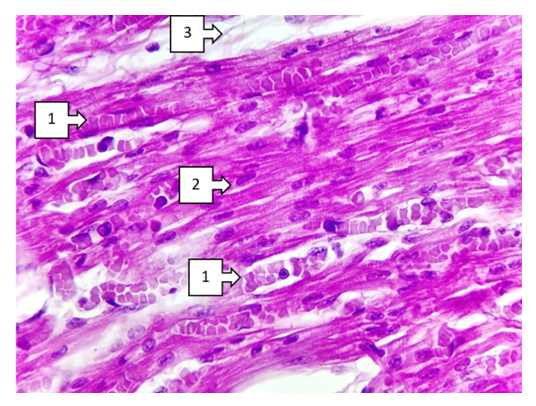
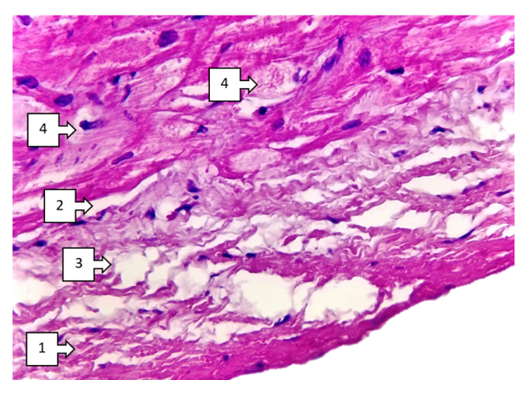
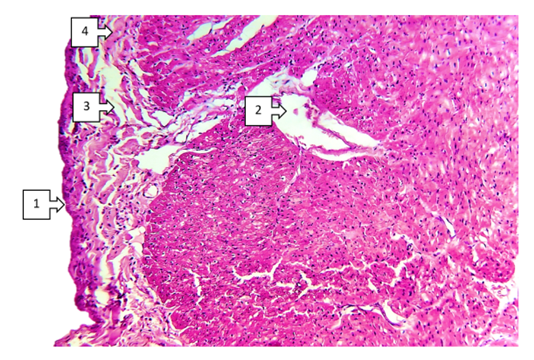
- When discussing the studied data on morphological changes of the right ventricle of the heart in Fallot's tetralogy, the following was established. Group hypertrophy of cardiomyocytes of the right ventricular myocardium was predominantly detected in the area of the anterior wall of the ventricle. Including branched hypertrophy of cardiomyocytes, large hyperchromic, well-defined cells of transverse extension were detected in the field of view 200x. The number of large cardiomyocytes was 220-255 in the field of view 200x. In comparison with the control group it was 2.25 times (up to 100-125), the size increased 2.5 times. Vessels between cardiomyocytes: capillaries and small calibre vessels were 1,75 times less than in the control group. These changes mean that compensatory mechanisms in the right heart sections are clearly developed. It was found that a large number of pale pink inclusions (glycogen) were found in the cytoplasm of atypical cardiomyocytes (pacemaker cells) located along the perimeter of the right ventricle. A cavernous appearance of lymphatic vessels of different width with cardiomyocyte inclusions was detected (see Fig. 1).
4. Conclusions
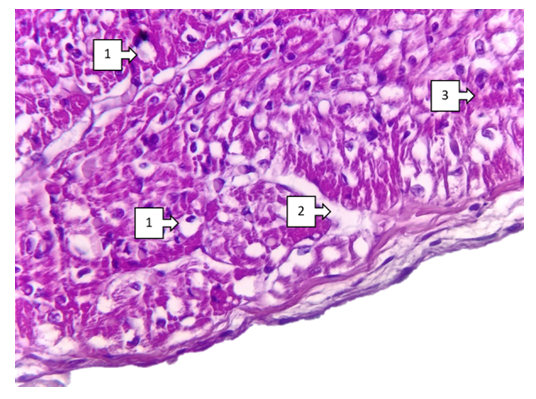
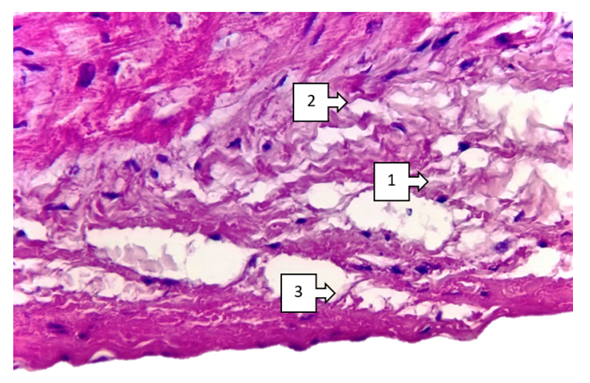
- Thus, characteristic aspects of morphological changes occurring in congenital heart defects, focal hypertrophy of cardiomyocytes, sclerotic changes around vessels, interstitial oedema and foci of fibroestosis have been determined. The most pronounced changes include focal endocardial thickening, group atrophic changes in subendocardial cardiomyocytes, lipomatous foci in the pericardium, and medium to small fatty dystrophic changes in right ventricular cardiomyocytes. These changes are determined in Fallot's tetralogy, among the combined types of congenital heart disease, in defects of the interventricular barrier, in transposition of the main vessels of the heart. These changes manifest themselves differently in different parts of the heart (frontal, inferior and interventricular septum) depending on the localisation of congenital heart defects. It has been established that the main part of cardiomyodestructive changes falls on the right ventricle in the blue type of heart defects from the clinical and morphological point of view of most congenital heart defects. These changes continue with the development of chronic venous stasis in the great circle of circulation. As a result, the heart lesion ends with the rapid development of right ventricular failure.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML