-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2023; 13(11): 1635-1642
doi:10.5923/j.ajmms.20231311.09
Received: Oct. 8, 2023; Accepted: Oct. 27, 2023; Published: Nov. 2, 2023

Association Between IL-10 Rs1800896, TLR 9 Rs5743836, and TNFα Rs1800629 Genes Polymorphisms and Blepharoconjuctivitis of Different Genes
H. Y. Karimov 1, Н. М. Kamilov 2, Sh. Sh. Nurmatov 3, Sh. R. Abdullaev 2
1Department of Molecular Medicine and Cell Technologies, Laboratory of Medical Genetics, Research Institute of Hematology and Blood Transfusion, Ministry of Health of the Republic of Uzbekistan, Tashkent, Uzbekistan
2Center for the Development of Professional Qualifications of Medical Workers, Tashkent, Uzbekistan
3Republic Hospital of Eye Diseases, Tashkent, Uzbekistan
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

There are several causes of vision system disorders and blindness, among which inflammatory diseases of the eye occupy the main place. The mechanism of the origin of inflammatory diseases in the eye area has not been thoroughly studied, and in this case, it occurs as a result of the complex effect of factors such as bacterial toxins, mechanical damage and immune dysfunction. Therefore, it is of great practical importance to identify polymorphisms that affect the expression and normal activity of TLR9, the proinflammatory cytokine TNFα and the anti-inflammatory IL10, which are important in the development of the innate immune response in the study of the mechanism of the development of inflammatory diseases of the visual system. it is possible to draw a conclusion about the relationship between genetic markers.
Keywords: TLR9, rs5743836, TNFα, rs1800629, IL-10, rs1800896, Blepharoconjunctivitis, Demodectic Blepharoconjunctivitis
Cite this paper: H. Y. Karimov , Н. М. Kamilov , Sh. Sh. Nurmatov , Sh. R. Abdullaev , Association Between IL-10 Rs1800896, TLR 9 Rs5743836, and TNFα Rs1800629 Genes Polymorphisms and Blepharoconjuctivitis of Different Genes, American Journal of Medicine and Medical Sciences, Vol. 13 No. 11, 2023, pp. 1635-1642. doi: 10.5923/j.ajmms.20231311.09.
1. Introduction
- Blepharoconjunctivitis - blepharitis and conjunctivitis are mutually complex manifestations and belong to the group of ophthalmological diseases. It is characterized by inflammation of the edge of the eyelids (blepharitis) and the surrounding conjunctiva (conjunctivitis). It is closely related to blepharitis and can be considered an advanced or aggravated form of blepharitis. If blepharitis is not treated in the early stages, the inflammation can spread to the nearby conjunctiva causing blepharoconjunctivitis [1]. It is difficult to distinguish the cause of blepharoconjunctivitis from the cause of blepharitis, which is related to the closeness of the eye structures and conjunctivitis in many cases also occurs together with blepharitis [2].Blepharoconjunctivitis can be divided into groups according to the clinical characteristics, in particular, the presence of diffuse ulcers in the marginal part of the eyelid is divided into types of infectious etiology, allergic etiology blepharoconjunctivitis with an acute onset without ulcers. But it should be noted that the absence of sores does not rule out infection. Acute infectious blepharitis can have a bacterial, viral or parasitic etiology [3]. According to the bacterial type, the most common blepharitis or blepharoconjunctivitis is blepharitis caused by staphylococcus.The development of staphylococcal blepharitis is considered to be due to inflammation of the eye surface with staphylococcal bacteria. However, the mechanism by which bacteria cause the symptoms of blepharitis is not fully understood. Comparison of ocular surface bacterial flora between healthy subjects and those diagnosed with staphylococcal blepharitis revealed some differences. In particular, only 8% of healthy examined patients were positive for Staphylococcus aureus, this indicator was found in 46-51% of patients diagnosed with staphylococcal blepharitis [4]. Because only half of patients diagnosed with staphylococcal blepharitis have positive cultures for S. aureus, additional contributing factors are thought to be present. Some researchers have suggested that the alteration may be caused by toxins produced by certain strains of S. aureus or S. epidermis [5]. 40% of patients with blepharitis have been found to have increased cellular immunity against S. aureus, a hyperergic immune response, and these patients often require topical corticosteroid therapy [6].Demodex parasites are also among the factors causing blepharitis and cause specific demodexosis blepharoconjunctivitis [7]. Blepharitis is thought to be caused by factors and wastes produced by demodex mites in the eyelash follicles and sebaceous glands of the eyelid causing the follicles and glands to become blocked and/or an inflammatory response [8]. In addition, demodex mites can cause blepharitis by carrying with them various bacteria such as streptococci and staphylococci [9], in which immune reactions are involved. In addition, it has been found that the bacteria inside the demodex mites can exacerbate blepharitis by inducing an immune response. Bacillus oleronius found in demodex mites has been found to have a proliferative effect on mononuclear cells in the peripheral blood of patients. Thus, demodex mites play an important role in the pathogenesis of demodex blepharoconjunctivitis by modulating the host's immune response by inducing hypersensitivity [10,11].The purpose of the work: to analyze IL-10 gene rs1800896 polymorphism, TLR 9 gene rs5743836 polymorphism, and TNF-A gene rs1800629 polymorphism in patients with blepharitis or blepharoconjunctivitis of different genesis.
2. Material and Methods
- 78 patients with blepharitis or blepharoconjunctivitis of various genesis were examined for the purpose of scientific research and they formed the main group. Also, for the purpose of comparison, 50 conditionally healthy donors without blepharitis or blepharoconjunctivitis were examined. Blepharitis or blepharoconjunctivitis was detected in the examined patients using the 10th revision of the manual "International Classification of Diseases" (2019 - https://mkb-10.com/index.php?pid=6052). According to it, chronic blepharoconjunctivitis occurred in 12 (15.4%) patients, blepharitis in 13 (16.7%) patients, acute inflammatory blepharoconjunctivitis and conjunctivitis in a total of 23 (29.5%) patients, demodectic blepharoconjunctivitis in 23 (29.5%) patients. in patients and non-specific conjunctivitis was found in 7 (9%) patients. The purpose of dividing the groups in this order was not only to determine the relationship between the tested gene polymorphisms and general blepharoconjunctivitis disease, but also to compare them with specific genesis blepharoconjunctivitis diseases and to determine which of the tested polymorphisms increase the risk of blepharoconjunctivitis development.In a clinical study, IL-10 gene rs1800896 polymorphism, TLR 9 gene rs5743836 polymorphism, and TNF-A gene rs1800629 polymorphism were examined in the blood of 78 patients with blepharoconjunctivitis. In the detection of IL-10 gene rs1800896 polymorphism, TLR 9 gene rs5743836 polymorphism, and TNF-A gene rs1800629 polymorphism in the venous blood of patients, nucleotide sequencing was performed using polymerase chain reaction on a DT-Lite 48 amplifier, using DNA-technology (Russia) reagents. Specific statistical processing was performed on the obtained results.
3. Results
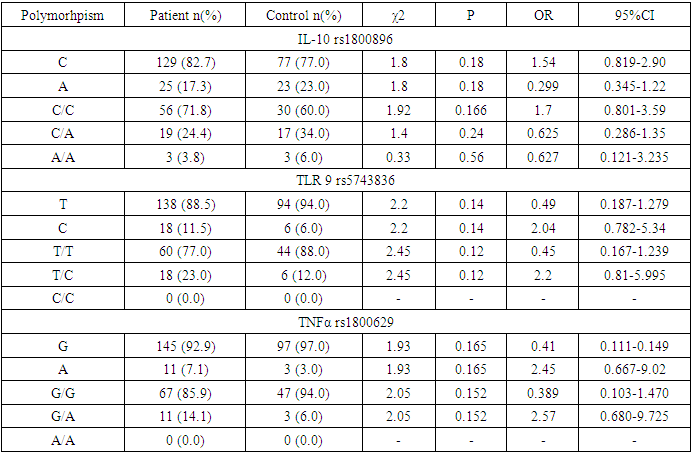
- According to the examined polymorphisms, the IL-10 gene rs1800896 polymorphism in the main and control group was homozygous wild, heterozygous and homozygous mutant genotypes, respectively, 44 (71.8%) and 30 (60.0%); 18 (24.0%) and 17 (34.0%); 3 (3.8%) and 3 (6.0%) were detected in subjects, and in TLR 9 gene rs5743836 polymorphism, homozygous wild and heterozygous genotypes were 57 (77.0%) and 44 (88.0%); 8 (23.0%) and 6 (12.0%) patients with a homozygous mutant genotype were identified in the main and control groups, and 55 (85.9%) and 47 (94.0) patients with a homozygous wild-type and heterozygous genotype for the TNF-A gene rs1800629 polymorphism, respectively. ; 10 (14.0%) and 3 (6.0%) were obtained (see Table 1).
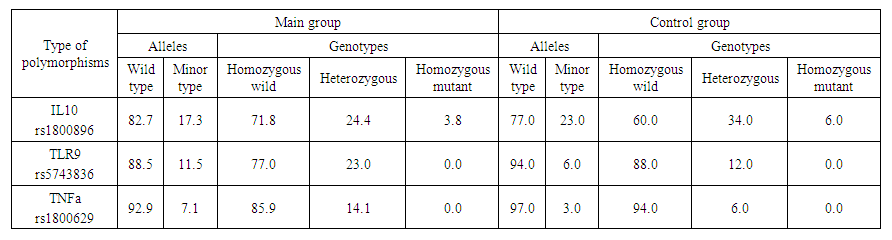 | Table 1. Percentage distribution of alleles and genotypes in the main and control groups |
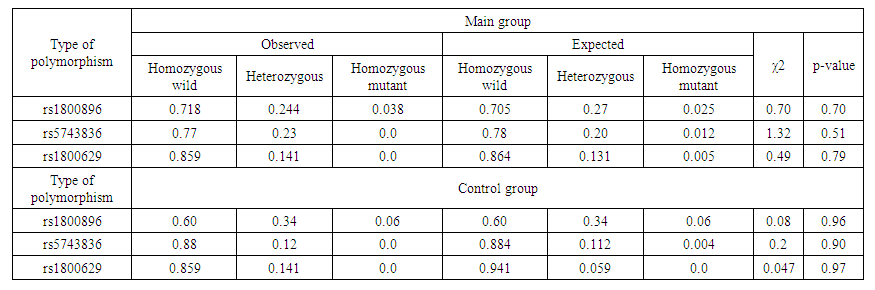 | Table 2. Comparison of the empirical results determined in the polymorphisms investigated in the main and control groups with the theoretical - expected results calculated by the Hardy-Weinberg law |
|
|
|
|
4. Discussion
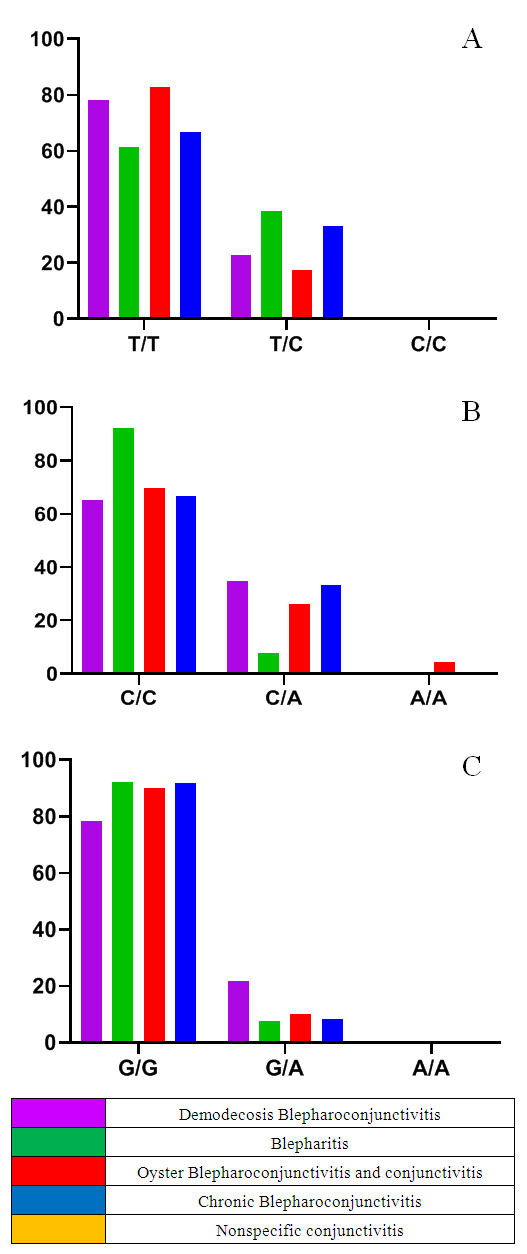
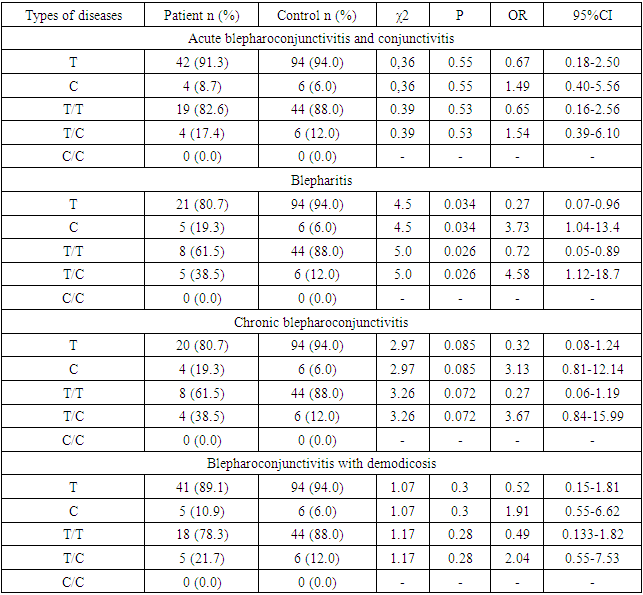
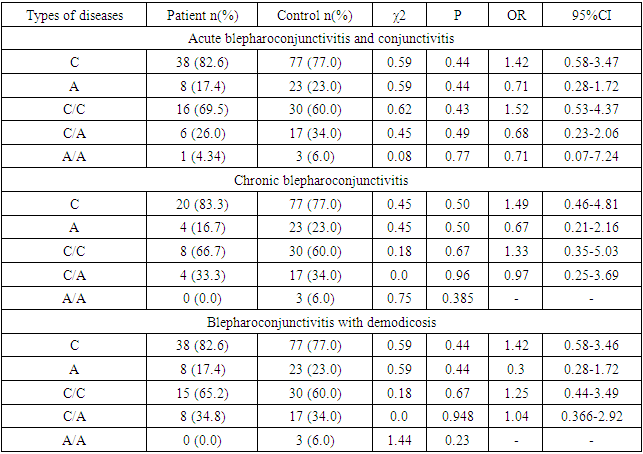
- The results of various studies were studied in order to gain a deeper understanding of the mechanism of origin of the results determined by the investigated polymorphisms during the research.TLRs (Toll-like receptors) are a family of receptors (PRRs) that recognize specific sequences on microorganisms and damaged cells, and play an important role in the activation of the innate immune system [11]. Studies have shown that microbial infection activates TLRs, and the interaction between TLRs and molecular sequences - PAMPs - promotes the induction of antimicrobial immunity [12] and the development of acquired immunity [13]. The protein encoded by the TLR9 gene is a member of the TLR family, which plays a key role in detecting various pathogens and activating innate immunity. They recognize pathogen-associated PAMPs expressed in infectious agents and induce the production of cytokines necessary for the development of innate immunity. Studies in mice and humans show that the TLR9 receptor promotes the innate immune response by recognizing unmethylated CpG dinucleotides in bacterial DNA [14].As a result of this change T1237C polymorphism of TLR9 gene, by changing the sequence of TLR9 protein, its affinity to the DNA of microorganisms and other ligands is impaired and the efficiency of innate immune response development decreases. For example, the T1237C polymorphism has been shown to increase the risk of developing malaria, mainly in children, due to impaired ability of microorganisms to recognize ligands [15].Similarly, in other studies, the TLR9 T1237C polymorphism may affect the normal functioning of the immune system by altering the production and activation of immune cells such as dendritic cells, macrophages, and T cells. For example, a study by Awastxi et al [16] showed that the TLR9 T1237C polymorphism was associated with impaired Th1 cytokine response in patients with rheumatoid arthritis. In a study by Leung et al [17], TLR9 T1237C polymorphism was associated with decreased dendritic cell activation and cytokine production in response to CpG DNA stimulation.The reason for this is probably due to the disruption of the normal activity of TLR9, which slows down the generation of an adequate immune response against pathogenic microorganisms, slows down their elimination, and creates favorable conditions for their development and deeper penetration into tissues and organs. Although, in our study, the association with TLR9 T1237C polymorphism was not determined in the main group of patients, when the main group was grouped according to the genesis of the disease, it was found that the minor allele S and T/S heterozygous genotypes were distributed in a statistically reliable high amount in patients with blepharitis disease (H01.0 – MKB10).The balance of pro-inflammatory and anti-inflammatory cytokines is important in order to clear the pathogen and limit the damage to tissues and organs of the host organism. In particular, pro-inflammatory cytokines (e.g., TNFα) mainly induce immune responses such as activation of macrophages, induction of apoptosis of damaged cells, and recruitment of additional immune cells, whereas anti-inflammatory cytokines (e.g., IL10) released from immune cells stimulate regulatory T cells and some macrophages. serves to suppress inflammation and immunity and enhances post-inflammatory regeneration and repair processes [18,19]. The balance of these cytokines can change at an inadequate time, creating conditions for the development of various chronic diseases and sepsis.In particular, patients with the minor allele of the TNFα G308A polymorphism are at increased risk of autoimmune diseases such as systemic lupus erythematosus, rheumatoid arthritis, or ankylosing spondylitis, and may be susceptible to certain infections [20]. This is because, as a result of TNFα G308A polymorphism, the G nucleotide in the promoter part of the gene is changed to A, which increases its affinity to transcription factors, which leads to an adequate level of TNFα in the plasma [21]. High concentrations of the cytokine TNFα can activate a variety of immune cells, particularly macrophages and T cells. This leads to the recruitment of more immune cells to the site of inflammation or tissue damage and, due to the establishment of positive feedback, greater and more sustained damage to normal cells than to altered cells and tissues by exposure to the phlogogenic agent. In chronic inflammatory or autoimmune diseases, as this population continues for a long time, chronic inflammation may develop in the tissues [22].In our study, although the correlation between TNFα G308A polymorphism and TNFα G308A polymorphism was not detected in the main group of patients, statistically reliable association was found between TNFα G308A polymorphism and demodectic blepharoconjunctivitis (B88.0 – MKB10) when patients in the main group were grouped according to the genesis of the disease. According to it, the development of demodectic blepharoconjunctivitis increased 4.35 (95%CI: 0.94-20.1) times in patients with heterozygous genotype of TNFα G308A polymorphism (χ2>3.84? p<0.05). In our opinion, the reason for this is that under the influence of various factors or directly under the influence of demodecosis parasites, the development of inflammation in the eyeball and the skin bordering on it creates conditions for the development of demodecosis parasites due to the fact that a high amount of TNFα cytokine (due to the A allele) increases the diademesis of various immune cells and damages normal tissue. must be related toOn the other hand, as mentioned above, the normal production and function of anti-inflammatory cytokines are important in the development of an adequate immune response, and their decreased expression can create conditions for the development of various autoimmune and chronic diseases. In particular, polymorphisms in the IL10 gene promoter region (in particular, G1082A) have been associated with the development of a number of diseases, including autoimmune, infectious diseases, cancer, Alzheimer's disease (AD), and lymphoblastic leukemia [23]. This is because the A allele of the G1082A polymorphism reduces IL10 expression compared to the G allele. That is, the A allele disrupts the binding of transcription factors that induce IL10 gene expression [24].However, in our study, no statistically significant association was found between patients and IL10 G1082A polymorphism in the main group and subgroups (χ2<3.84? p>0.05).
5. Conclusions
- According to the results of the study, no statistically reliable association was found between patients with blepharoconjunctivitis disease of different genesis (main group) and IL-10 gene rs1800896, TLR9 gene rs5743836 and TNFα gene rs1800629 polymorphisms (χ2<3.84? p>0, 05). On the other hand, when patients were grouped according to the genesis of the disease, a reliable association between TLR9 gene rs5743836 polymorphism and blepharitis was found (χ2=4.5? p=0.034), compared to patients with the heterozygous genotype, the disease development was 4.58 (95%CI: 1.12-18.7) times. increase was found. Thus, a statistically reliable correlation was found between TNFα gene rs1800629 polymorphism and the development of demodectic blepharoconjunctivitis (χ2=3.99? p=0.046), and the disease development increased 4.35 (95%CI: 0.94-20.1) times in patients with the heterozygous genotype.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML