Tursunov X. Z., Mallaev M. M.
Tashkent Medical Academy, Tashkent, Uzbekistan
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
The aim of our study to analyse the results of tumor microenviron in patients with gastric cancer. According to the results of our analysis of the pre-collected literature and independent research, the con-clusions of the molecular examination in gastric cancer and information on the microenvironment of the tumor have shown that it is possible to control the disease at any stage.
Keywords:
Gastric cancer, Monoclonal antibodies, Tumor morphology, Checkpoint inhibitors
Cite this paper: Tursunov X. Z., Mallaev M. M., Role of Molecular Feachures of Gastric Cancer, American Journal of Medicine and Medical Sciences, Vol. 13 No. 11, 2023, pp. 1595-1599. doi: 10.5923/j.ajmms.20231311.01.
1. Introduction
According to numerous studies. Today, many studies have confirmed that the risk potential of stomach cancer is related to its morphology and is widely used as one of the prognostic criteria for stomach cancer. At the same time, existing prognostic schemes are usually based on such characteristics as the degree of tumor differentiation, the depth of invasion, the type of growth, the degree of stroma formation and neo-angiogenesis, local immune responses.In recent studies, the level of risk of gastric cancer has been shown to depend on the functional characteristics of tumors. In this case, the prognosis is based on indications of immunohistochemical expression of mucins synthesized by the gastric mucosa. There are considerations that expression of functional activity markers by tumor cells does not affect its histological type, histological stages, and gastric localization of the tumor. Nevertheless, in a number of studies it has been proposed to divide gastric cancer into various IFA options based on a concentration of tumor cell expression mucins [1,2]. In a number of studies, it has been found that patients with different IFA variants of carcinoma also have different potentiation of viability and risk. Article analysis has shown that the levels of risk among the various IFA variants of gastric cancer are still disputed in studies that have been studied. Many of the researchers included the mixed IFA variant of gastric cancer among unsatisfactory prognostic factors, while carcinomas with gastric and intestinal variants were perceived as proportionately safer. However, several studies have found the worst prognosis in patients who have undergone surgery on the gastric or intestinal IFA variant of carcinoma. Thus, in gastric cancer, the question remains open about the potential level of danger of each IFA options [3].In the available literature, there is no systematic data on the rates and patterns of cellular renewal of gastric carcinomas. Also, the effect of these criteria on carcinoma risk potentiation and the prognosis of operated patients has not been studied. It is known that tumor cell proliferative activity and apoptosis death rates must be calculated to determine cell renewal rates. The number of studies aimed at solving this problem in gastric carcinomas is very small, and they mainly focus on one of the most important indicators, listed above [4].It should be noted that the expression of the most important molecular-biological markers involved in the development of tumors in gastric carcinomas has not been studied until the end, as well as their effect on tumor risk potentiation and prognosis. It should also be distinguished that studies investigating immunohistochemical detectable expression of e-CAD, COL4, TN-c, MMP2S in gastric carcinomas are very rare, as well as controversial. While the expression of mmp3 in this area has not been studied at all. In addition, the expression indicators of these listed markers and the IFA properties of gastric cancer have hardly been compared [5,6]. There are also no studies comparing the size of tumor infiltration and pathomorphological analysis of operating material of ”proximal" gastric cancer carcinomas, identified using magnetic resonance imaging [7,8]. Thus, we can see a number of areas of tumor cells, such as IFA specificities and invasive properties, cell renewal rates and patterns in gastric carcinomas, the cell external Matrix state of the tumor, as well as the clarification of the light, differential pathomorphological diagnosis and prognosis criteria of gastric cancer, in need of further complex research [9].The aim of our study to analyse the results of tumor microenviron in patients with gastric cancer.
2. Materials and Methods
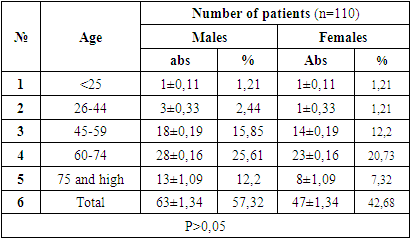
Based on the study objective, the age and gender-related aspects of the research object, differences in clinical course, the results of general clinical and special instrumental examination methods, the position of signalling pathways in disease development and the susceptibility of immunological indicators to change were studied.In the course of scientific work, the results of diagnostics and treatment of 110 patients with gastric cancer who received diagnostic and inpatient treatment at non-governmental medical institutions “Akfa Medline”, “new Life Medical” and “Mediofarm” were evaluated from 2017 to 2022. Due to the fact that our scientific work is intended to be carried out in the cohort method, the control group is not taken into account. As part of the planned scientific work, modern methods of research were used against the background of universally recognized research methods – molecular microenvironments of the disease were studied using cytogenetic, cytomorphological and immunohistochemical methods, and the effectiveness was applied to practice. The diagnosis of all patients is verified by histological method. The age of the core group of patients is 20 to 79 years, with an average age of 53 years. (Table 1). There were 62 males (57.32%) and 47 females (42.68%), giving a ratio of 1: 0.77.Table 1. The distribution of patients under study by age and gender
 |
| |
|
3. Results
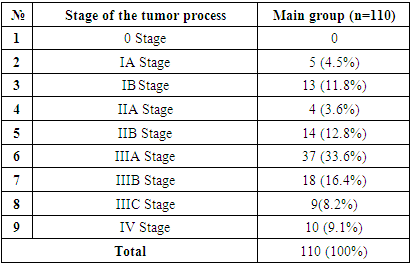
Gastric cancer staging is divided into stages by the AJCC (American Joint Committee on Cancer) based on its specially developed classification with the 8th edition of 2017. According to this classification, there are 4 levels of differentiation of gastric cancer into stages. The classification of their patients under study by the stages of the disease is presented in Table 2.Table 2. Distribution of patients by stage of the disease
 |
| |
|
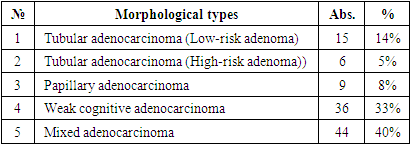
When we analyse the table above, we can see that the majority of patients are diagnosed in Stage III. We have analysed in our research results that show that this has a dramatic effect on treatment outcomes. In turn, comparing the histological properties of the post-Practical macro preparate of the initial endoscopic verification, found the effectiveness of information by elucidating the sensitivity of instrumental investigations in the cross-section of histological variations. In particular, the distribution of endoscopic bioptates obtained during the period of appeal of patients with gastric cancer according to the degree of histological differentiation is presented in Table 3.Table 3. Distribution of patients with gastric cancer by tumor morphology (JSST 2019) (n=110)
 |
| |
|
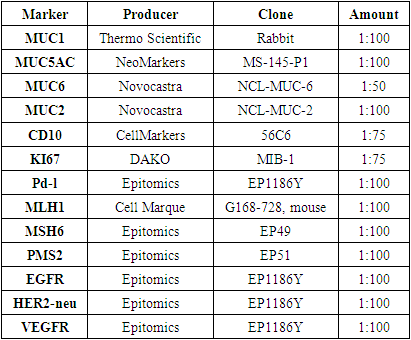
When the results obtained are estimated, the occurrence of weak cognitive and mixed adenocarcinomas in gastric cancer has the greatest indicator, which is a morphological unit that later requires a deeper examination and immunohistochemical confirmation. Other morphological types of Ham are present in gastric cancer, which make up very few percentages in terms of occurrence.We divided the bioptats from patients into types according to the histological manifestations in question when dividing them into the above histological types:1) mainly round-core cells (similar in morphological appearance to small lymphocytes: with a clear nuclear border, with a small dispersal chromatin, no nucleoli are visible, the cytoplasm of cells is thin, with an indistinct nuclear periphery outlines);2) cells with centrocyte morphology (are small cells with a divided nucleus, a slightly dispersive or granular chromatin, a nucleus that is not clearly visible, and a mid-width colon cytoplasm);3) cells of mixed morphology: cells with a rounded and centrocycymon nucleus.In order to determine the degree of occurrence of the pathological process and the width of the affected area in the anatomical parts of the stomach, patients were divided by the location of the tumor and the area of damage to the organ. In doing so, data was collected by studying the results and conclusions obtained during the endoscopic examination process, and post-Practical macro-preparation.For immunohistochemical examination, the most representative regions of the tumor were selected and serial paraffin incisions 5 µm thick were prepared on 15-20 item stools. The reaction was carried out with the peroxidase – anti-peroxidase method on the standard protocol, in which the monoclonal antibodies (Table 4). The study protocol was approved by the ethics committees of all participating clinical centers. Table 4. Monoclonal antibodies used in the study
 |
| |
|
After processing the cuts in the microwave Mode (2 minutes interval and 2 times 5 minutes with a power of 650 Watts), incubation with primary antibodies was carried out. Later stages used diaminobenzidine chromogen from KIT (EnVision, Mouse/Rabbit) and the Daco enterprise, while staining the cell nucleus with Maer hematoxylin. Gastric carcinomas can measure immunohistochemical reactions of tumor cells and the non-tumor epithelium of the mucous membrane with molecular-biological markers, and it is also used as a positive control. In parallel cross-sections of the same block, antibodies can be substituted as a negative control if a buffer mixture is used instead. Marker expression was evaluated in promils, by the number of immune-positive tumor cells in every 1,000 cells in the X400 magnified representative fields of the microscope. When >50% of tumor cells (their nuclei for Ki67) or CD10 solitary cells were stained, the immunohistochemical reaction was perceived as positive.Tumors have been conditionally classified into high, medium, and weak immunoreactive types to semi-assess the expression of mucins and CD10 of tumor cells in gastric carcinomas. In the first group – markers, the number of tumor cells with immune-positiveness was 50-250‰, in the second group – 250-500‰, in the third – carcinomas with an immune-positiveness of 500‰ and above. Moderate to strong immunoreactivity has been distinguished in the evaluation of MMP2 and MMP3 expression. In the first group, the number of tumor cells immune-positive to MMP2 was ≤400‰, while in the second it is higher than 400‰. In mmp3, however, ≤500‰ and >500‰ respectively. Cell renewal parameters of gastric carcinomas and tumor-free mucosa have been studied on quantitative indicators of cell death and proliferative activity, using methods for detecting MI and AI, as well as Ki67s. In doing so, the calculation of KI67-labeled nuclei, mitotic forms, and apoptotic cells was done by taking into account no less than 1,000 tumor cells in the maximum number of Representative visual fields when the microscope was magnified x1000. The results are reflected in promille. PD-L1 state studies have a signal enhancer using PD-L1 SP142 antibodies by immunohistochemical method (Ventana Medical Systems, Inc., USA) through the Opti View DAB IHC Detection Kit detection system. Two incisions were made from each tumor sample: one for the application of primary antibodies and the other for negative control. Samples of cell samples (NCL-h226 - positive cell line and MCF-7 - negative cell line) of vesicles, tonsils and satellite tissue were used as a control protocol of the study in each reaction cycle. A semi-automated immunohistochemical method has been used for all antibodies. Based on antibodies as well as NordiQC external quality control applications, the SP142 antibody (Ventana Medical Systems, Inc., USA) to perform the reaction with the VENTANA Bench Mark ULTRA immunehistotainer (Ventana Medical Systems, Inc., USA) applied. The tumor PD-L1 state was evaluated on the basis of the IC assessment system: the ratio of the area occupied by immune cells representing PD-L1 to the area of all living tumor cells, microenvironment immune cells and granulomas, which, multiplied by 100 and expressed as a percentage, has a threshold value of 5% or more. In accordance with the recommendations for assessing the results of PD-L1 expression, the ability to stain membranes in tumor and immune cells, regardless of intensity, was evaluated.The study of the MSI phenotype was carried out using the following antibodies that work with the immunohistochemical method: MLH1 (Clone ES05); MSH6 (Clone EP49); PMS2 (Clone EP51). The incubation time with primary antibodies was 30 minutes. The detection system is EnVision FLEX for the Dako Autostainer Link 48 (a company of Dako An Agilent Technologies). Diaminobenzidine (DAB) was used as a chromogen. Demasking was done on a buffer with a pH of 9.0 in the preprocessing module (PTModule) for the Dako Autostainer Link 48 immuno-autostainer, under a temperature of 97°C, for 25 minutes.In the absence of nuclear immunohistochemical staining of at least one marker, a positive MSI phenotype was diagnosed. The intensity of immunohistochemical staining of the nucleus was assessed using a 3 – point system: 0 - staining was not observed; 1 - light staining; 2 - moderately observed staining; 3-strong bright staining (Figure 1). According to how much percent of the entire tumor area is covered with painted nuclei, all cases are divided into four groups: Group 1-not observed at all or less than 1%; Group 2-from 1% to 10%; group 3-from 11% to 49%; Group 4-from 50% to 100%.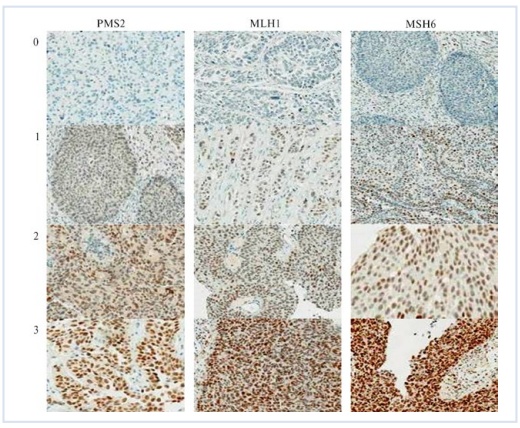 | Figure 1. The intensity of immunohistochemical staining |
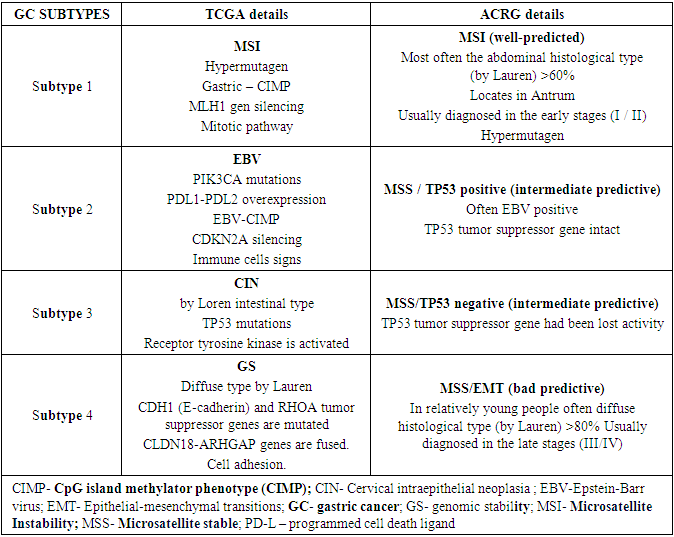
The expression of vascular endothelial growth factor (VEGFR) and epidermal growth factor (EGFR) receptors was studied according to a standard methodology, using the immunohistochemical method through the semiautomatic immunohistin Bond-maX (Leica Biosystems, Germany). The study used Epitomics enterprise antibodies. The numerous investigations recommend the use of molecular classification in gastric adenocarcinoma. One similar scientific organization, TCGA (the cancer genomic atlas), distinguishes four genotypes, molecular in gastric adenocarcinomas, which are EBV-induced tumors, MSI active tumors, genetic stable (GS) tumors, and chromosomal non-stable tumors (SIN). But there is not enough information about the clinic, prognosis and similar characteristics of the disease in these subtypes, but it is known that the gastric cancer subtype corresponds to the diffuse histological type in the Lauren classification, while the rest of the subtypes often correspond to the intestinal histological type.Another scientific investigation team (ACRG) found that since the disease can be divided into 4 different genotypes, they are MSS as well as tumors with unchanged TP53 gene (MSS/TP53+); MSI active tumors; MSS as well as epithelial-mesenchymal transitory (MSS/EMT) tumors; MSS as well as tumors with mutated TP53 gene (MSS/TP53 -). This classification provides valuable information on the diversity of biological properties among gastric adenocarcinomas. This is not a clear pathology of gastric adenocarcinomas, but the fact that different subtypes of the disease occur according to the nature of the mutation in patients, and it is this issue that serves as a testament to the identification of new healing agents now and in the future. HER2 is currently the only biomarker in target treatment in patients isolated into specific groups.After all, despite the different approach and terminology applied, the ACRG team had almost (incomplete) repeated the molecular classification of TCGA. While this is the case, ACRG is characterized by having relatively more clinical cases. Patients with MSI-active or EBV-induced tumors in particular have been observed to have greater survival compared to those with MSS/EMT subtypes. Disease recurrence has also been studied for its diversity in molecular subtypes, for example in MSS/EMT subtype patients with a high recurrence characteristic (63% to 23%) as well as a high peritoneal metastasis risk compared to MSI-active subtype patients. The molecular classification of the disease is structured according to the result of mutation of different genes, below we will get acquainted with the genes that lead to pathology and their mutations.Table 5. Expression of PD-L1 and PD-1 in gastric adenocarcinomas and its analysis
 |
| |
|
For the first time in Uzbekistan, we studied the expression of PD-L1 and PD-1 using immunohistochemistry in a cohort consisting of 70 samples and 15 related liver metastases of patients with gastric cancer. Expression of PD-L1 has been found in 21 gastric cancers (30.1%) and 9 hepatic metastases (60%) tumor cells, and 62 gastric cancers (88.4%) and 11 hepatic metastases (73.3%) immune cells. PD-1 expressed in tumor infiltrating lymphocytes, 37 in gastric cancer (52.8%), and 11 in liver metastasis (73.3%). Expression of PD-L1 is significantly more common in men, proximal gastric cancer, unclassified, Her2 / neu-positive, Epstein-Barr virus, and microsatellite unstable gastric cancer. High expression of PD-L1 / PD-1 showed better patient survival and disease prognosis. The correlation of PD-L1/PD-1 expression with various clinical and pathological features of the patient can serve as a surrogate marker for PD-L1-positive gastric cancer and determine the use of immune checkpoint treatment strategies.
4. Discussion
The genetic complexity of gastric cancer has recently been demonstrated in whole genome sequence analysis. A molecular classification has been proposed that classifies four subtypes: positive Epstein-Barr virus (EBV), Microsatellite Instability (MSI), chromosome unstable, and genomically stable stomach cancer [10,11]. These current results serve as a roadmap for patient stratification and targeted treatment trials, and PD-L1 has been found to be exaggerated in EBV-positive and MSI GCS.Currently, more than 400 studies worldwide focus on the PD-L1 / PD-1 immune checkpoint signalling pathway, including 65 gastrointestinal cancer studies and there is some evidence that PD-L1 expression is associated with PD-L1 / PD-inhibition. 1 signalling system within cancer cells [8]. Early results of metastatic gastric cancer with PD-1 / PD-L1 checkpoint inhibitors were very promising, with Phase III studies beginning recently. To date, PD-L1/PD-1 in stomach cancer has only been evaluated in small cohorts of Asian patients, Caucasians. In all patients in the previous groups, gastric cancers are known to contain different gene signatures [12]. Thus, data on the effects and effects of PD-L1 / PD-1 on stomach cancer in patients in our country were systematically studied for the first time. To fill this information gap, we regularly examined the expressions PD-L1 and PD-1 in a cohort that is small and meticulously described in Uzbekistan.The clinical and pathological features of our cohort of patients were observed in 70 patients. Overall survival data was available in 68 (97.1%) cases, while tumor-specific survival data was available in 63 (90.0%) cases. The average observation was 9.2 months (0.5 to 24 months).
5. Conclusions
According to the results of our analysis of the pre-collected literature and independent research, the conclusions of the molecular examination in gastric cancer and information on the microenvironment of the tumor have shown that it is possible to control the disease at any stage. The results obtained during our research literature didn’t differ dramatically from the data in the analyzes. This shows that the use of European-standard treatments in patients with gastric cancer living in the region of Uzbekistan provides effective results.
References
| [1] | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019; 69(1): 7–34. |
| [2] | Vedeld HM, Goel A, Lind GE. Epigenetic biomarkers in gastrointestinal cancers: The current state and clinical perspectives. Semin Cancer Biol. 2018 Aug; 51: 36-49. |
| [3] | Tahara T, Arisawa T. DNA methylation as a molecular biomarker in gastric cancer. Epigenomics. 2015; 7(3): 475-86. |
| [4] | Abu-Sbeih H, Wang Y. Gastrointestinal Tract Adverse Events. Adv Exp Med Biol. 2020; 1244: 247-253. |
| [5] | Yoshida S, Yamashita S, Niwa T, Mori A, Ito S, Ichinose M, Ushijima T. Epigenetic inactivation of FAT4 contributes to gastric field cancerization. Gastric Cancer. 2017 Jan; 20(1): 136-145. |
| [6] | Li XP, Qu J, Teng XQ, Zhuang HH. The Emerging Role of Super-enhancers as Therapeutic Targets in The Digestive System Tumors. Int J Biol Sci. 2023 Jan 22; 19(4): 1036-1048. |
| [7] | Grady WM, Yu M, Markowitz SD. Epigenetic Alterations in the Gastrointestinal Tract: Current and Emerging Use for Biomarkers of Cancer. Gastroenterology. 2021 Feb; 160(3): 690-709. |
| [8] | Peng Z, Cheng S, Kou Y, Wang Z. et al. The Gut Microbiome Is Associated with Clinical Response to Anti-PD-1/PD-L1 Immunotherapy in Gastrointestinal Cancer. Cancer Immunol Res. 2020 Oct; 8(10): 1251-1261. |
| [9] | Chen K, Wang X, Yang L, Chen Z. The Anti-PD-1/PD-L1 Immunotherapy for Gastric Esophageal Cancer: A Systematic Review and Meta-Analysis and Literature Review. Cancer Control. 2021 Jan-Dec; 28: 1073274821997430. |
| [10] | Sasaki S, Nishikawa J, Sakai K. EBV-associated gastric cancer evades T-cell immunity by PD-1/PD-L1 interactions. Gastric Cancer. 2019 May; 22(3): 486-496. |
| [11] | Yang J, Liu Z, Zeng B, Hu G, Gan R. Epstein-Barr virus-associated gastric cancer: A distinct subtype. Cancer Lett. 2020 Dec 28; 495: 191-199. |
| [12] | Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H. Gastric cancer. Lancet. 2016; 388: 2654–64. |



 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML



