Shukurova Lobar Khusanovna1, Daminova Lola Turgunpulatovna2, Buranov Sherzod Mizrobovich3
1Senior Lecturer, Tashkent State Dental Institute, Tashkent, Uzbekistan
2D.m.s., Professor, Tashkent State Dental Institute, Tashkent, Uzbekistan
3Doctor, Hematologist, Republican Medical Center of Hematology, Tashkent, Uzbekistan
Correspondence to: Shukurova Lobar Khusanovna, Senior Lecturer, Tashkent State Dental Institute, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
Chronic kidney disease (CKD) is one of the most pressing problem of today and requires a lot of attention. The reason is that it leads to early disability of the population, as well as it increases of mortality of patients from this. In the II-III stages of CKD, the main role is played by the functional state of the kidneys and violations of blood rheology, which affects the course of the disease, the development of complications, decreases the quality of life of patients, early initiation of renal replacement therapy. Currently, experimental and clinical data have been obtained, which show that one of the important mechanisms of pathogenesis in kidney functionalization may be disorders in the coagulation system (hemostasis) both locally in the kidneys and with the capture of the microcirculatory bed of other organs. The most pronounced changes in the hemostasis system with impaired renal function are noted in diabetic nephropathy, mixed form of chronic glomerulonephritis (GN), nephrotic syndrome (NS), hematuric variant of chronic nephritis, nephropathy of pregnant women, lupus nephritis, lipoid nephrosis, acute glomeulonephritis.
Keywords:
Chronic kidney disease, Platelet aggregation, Blood rheology, Coagulation system
Cite this paper: Shukurova Lobar Khusanovna, Daminova Lola Turgunpulatovna, Buranov Sherzod Mizrobovich, Effects of Calcium Dobesilate on Platelet Aggregation and Blood Rheology in Patients with Chronic Kidney Disease, American Journal of Medicine and Medical Sciences, Vol. 13 No. 10, 2023, pp. 1488-1495. doi: 10.5923/j.ajmms.20231310.27.
1. Introduction
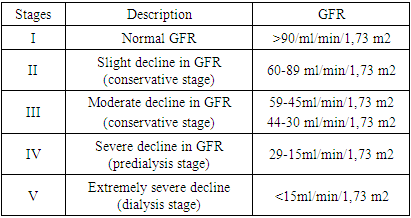
Chronic kidney disease (CKD), which the experts of the Advisory Council, initiators of the quality of treatment of kidney disease of the (K/DOQI) National Kidney Foundation of the USA define as the presence of kidney damage or a reduced level of kidney function for at least 3 months, regardless of etiology, is an acute medical and social problem related to the priorities of national health systems in all industrialized countries of the world, where over the past decades, there has been a steady increase in the prevalence of chronic renal failure (CRF) associated with a decrease in the quality of life of patients and a high mortality rate [2]. The growing population of patients with chronic kidney disease (CKD) is becoming an urgent health problem of the XXI century in all, especially developed countries of the world. The results of epidemiological studies indicate that even the earliest subclinical renal dysfunction are independent risk factors for cardiovascular events and death [1]. The prevalence of CKD in the world is also difficult to assess, since different criteria and assessment methods are used. The prevalence of CKD in Romania (where 60,969 people aged 18 and older were surveyed) is 7% [7], in the USA among the population aged 20 and older – 12% [3] higher figures are given, in particular in the USA, which largely depends on the assessment methodology [4].In 2005, the most authoritative organization — KDIGO (Kidney Diseases: Improving Global Outcomes) — confirmed the initiative of K/DOQI to widely use the term CKD. CKD is not classified in the ICD-10. However, in the international classification ICD-9-CM, since October 1, 2005, all five stages of CKD have been assigned their own codes. The criteria for determining CKD in adults and children are identical. Classification of chronic kidney disease (Table 1).Table 1. STAGES OF CHRONIC KIDNEY DISEASE according to NRF/KDOQI (National Kidney Foundation/Kidney Disease Outcomes Quality Initiative, 2002)
 |
| |
|
CKD can be both an independent diagnosis and a generalizing term. For example, if a urinary syndrome is detected for the first time — proteinuria or erythrocyturia and this urinary syndrome has been observed for more than three months, then CKD can be considered as the primary diagnosis. One of the most controversial issues is the involvement of the vascular endothelium. Normally, vascular endothelial cells have high antiaggregational, anticoagulant and fibrinolytic activity. In patients with chronic kidney disease, a decrease in the antithrombogenic activity of the vascular wall was found. [3] A decrease in the antiaggregational and fibrinolytic activity of the vascular wall was recorded in chronic kidney disease in all stages. [4] It has been shown that endothelial-platelet dysfunction develops in patients with chronic kidney disease. In patients with chronic kidney disease at all stages of the disease, a persistent increase in the Willebrand factor was recorded in the blood of patients, which indicated a violation of the functional properties of the vascular endothelium. A local increase in endothelin synthesis1 was noted in the kidneys, which, being a vasoconstrictor, causes an increase in peripheral vascular resistance, a decrease in renal blood flow and a decrease in glomerular filtration rate. An increase in the Willebrand factor provokes adhesion, agglutination of platelets and their aggregation; at the same time, blood platelets of patients with various stages of chronic kidney disease reacted differently to stimuli. When TIC is attached, platelet aggregation processes begin to prevail. E.A. Movchan also cites the results of experimental studies demonstrating that activated platelets provoke mesangial proliferation. In addition, CKD (chronic kidney disease) shows a change in the concentration of platelet secretion products, especially thromboxane A2 and lamellar factor 4 (PF4) in the glomerular zone and interstitium. PF4 has a wide spectrum of action, participating in the reactions of neutrophil chemotaxis and their activation, and is also able to integrate into the basement membrane of the kidneys, thereby disrupting the permeability of the glomeruli. An increase in thromboxane A2 was detected in patients with glomerulonephritis in all phases of its course (active, inactive, attachment of the tubulo-interstitial component - TIC). The involvement of thromboxane A2 and PF4 in inflammation and fibrosis of renal tissue is discussed. (5) An increase in tissue factor and factor VII, the number of 1 + 2 prothrombin fragments was found with a significant decrease in the content of antithrombin III, factor X and the ratio of free protein S to protein S. The change in these indicators correlated with the degree of renal dysfunction. These results, according to the authors, demonstrated that the development of hypercoagulation was independent of endothelial function, but was associated with the inflammatory process.The early stages of CKD (I-III) do not appear in any of the modern scales. At the same time, it is well known that with the progression of CKD and its combination with AF, the risks of serious bleeding, strokes and other thromboembolic complications (TEC), any coronary events significantly increase, and overall mortality increases. Chronic kidney disease significantly changes the pharmacokinetics of drugs excreted by the kidneys, to which all anticoagulants belong to one degree or another. This fact significantly complicates the selection of adequate anticoagulant therapy in this cohort of patients, and many simply do not receive it due to fear of the development of undesirable side effects. Nevertheless, the absolute majority of patients with CKD need anticoagulant therapy to prevent life-threatening complications of AF. In such conditions, it is necessary to “balance” when choosing both the drugs themselves and their doses, so as not to harm the patient. [8]Calcium dobesilate (lat. Calcii dobesilas, systematic name — calcium 2,5-dihydroxybenzenesulfonate, also calcium 2,5-dioxybenzenesulfonate, also calcium 2,5-dihydroxybenzenesulfonate) — calcium salt of dobesilic acid. In practice, it is necessary to use this drug as reducing blood clotting and for the prevention of atherothrombosis and venous thromboembolism. Calcium dobesilate can reduce platelet aggregation, plasma and whole blood viscosity, reduce fibrinogen levels, affect the function of endothelial cells by stimulating the release of tissue plasminogen activator and increasing blood fibrinolytic activity [5]. These properties of the drug potentiate the action of antiplatelet agents and anticoagulants, which can help reduce their dose during the course of treatment with calcium dobesilate and reduce side effects. On the other hand, additional control of efficacy and safety markers is required, in particular, it is advisable to clarify the value of the international normalized ratio with the combined use of warfarin and calcium dobesilate, as well as the measurement of activated partial thromboplastin time with the combined use of calcium dobesilate and unfractionated heparin.In addition, a wide range of prescriptions is explained by the pharmacodynamics of calcium dobezilate (angioprotector, reduces increased vascular permeability, increases resistance of capillary walls, improves microcirculation and drainage function of lymphatic vessels, moderately reduces platelet aggregation and blood viscosity, increases the elasticity of the erythrocyte membrane).
2. The Purpose of the Study
To study the effects of the drug calcium dobezilate on platelet aggregation, blood rheology in patients with CKD diabetic and non-diabetic etiology of stage II-III.
3. Materials and Methods of the Study
The study was based on clinical materials, laboratory analyses and instrumental studies of 110 patients undergoing treatment in the nephrology department of the multidisciplinary TMA clinic. Patients with stage II-III CKD were selected (according to creatinine 89-30 ml/min). The duration of the study was 90 days. All patients were conditionally divided into 2 groups. For the study, 110 patients with CKD which was caused by primary and secondary nephropathies that developed on the basis of various renal and some non-renal deseases by creatinine GFR 45-89 ml/min/m2 were received treatment in the nephrological department of the Tashkent Medical Academy.According to etiology, the large part of patients are patients with a diagnosis chronic glomerulonephritis - 74, 10 of them - chronic pyelonephritis. The nosology of chronic pyelonephritis also includes secondary pyelonephritis caused by kidney stones and polycystic kidney disease. A number of other diseases were also included (5 chronic tubulointerstitial nephritis, 14 systemic diseases, 2 amyloidosis of the kidneys and 5 patients with diabetes mellitus).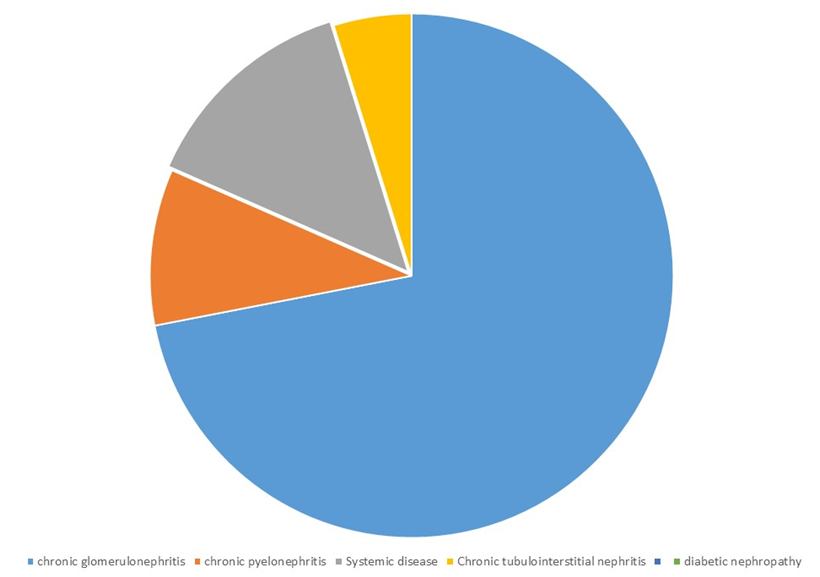 | Figure 1 |
At the same time, a group of diseases were not included to the study according to the following criteria: CKD occurring in hematological diseases associated with peptic ulcer of the gastrointestinal tract, other endocrine genesis, kidney tumors.In the first group, 55 patients were regularly given dipyridamole 75 mg 2 times a day in addition to basic therapy as antiplatelet therapy. In the second group, 65 patients underwent basic therapy and were prescribed calcium dobezilate (Doxy-Heme) 500 mg 3 times a day as anticoagulant, antiplatelet therapy. In both groups, general clinical (CBC, ESR, UA) and biochemical blood tests (urea, creatinine, total protein, ALT, AST, bilirubin), coagulogram, ADP were performed. Evaluation of the effectiveness of therapy was carried out according to the following parameters: 1. Rheological properties of blood (RPB) – viscosity of whole blood, plasma and suspension of erythrocytes with hematocrit 45% at shear rates of 3-300 s, platelet aggregation activity according to the G. Born method (1962) modified by Z.A. Gabbasov (1989). The induced (adenosine diphosphate – ADP, adrenaline, collagen) platelet aggregation was assessed. 2. Kidney function: MAU using test strips “Micral Test” (firm “La Roshe”, Switzerland), glomerular filtration rate (GFR) by endogenous creatinine clearance (Rehberg method).3. Peripheral vascular resistance: Dopplerographic test was performed on the equipment “Sonoscape S20 Color Doppler diagnostic”. With the help of this study, the spectral method was used to study the velocity and vascular resistance of blood flow in the main, arched, interstitial vessels of the kidney.
4. The Results of the Study and Their Discussion
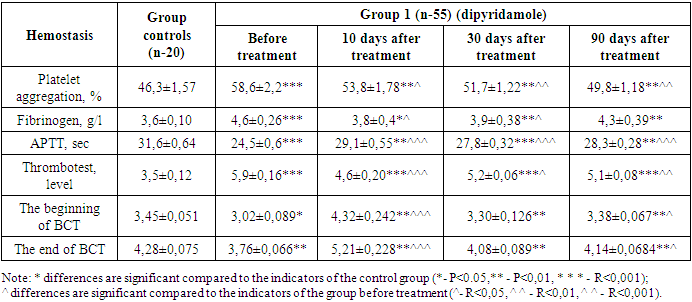
As the results of our study showed, all patients had clinical and laboratory signs of chronic kidney disease. Complaints included decreased diuresis and turbidity of urine, nocturia, headache and symptoms of general weakness. In this study, we studied the effect of calcium dobezelate as an anticoagulant and obtained coagulogram indicators (PTI, INR, Fibrinogen, PTT, Hematocrit, APTT) from the ADP hemostasis system, and to assess the functional state of the kidneys, we used indicators of biochemical blood analysis (urea, creatinine, glomerular filtration rate). When using the drug dipyridamole, the following results were taken according to these indicators: Table 2. Dynamics of changes in hemostasis indicators during treatment with antiplatelet agents in group 1 patients
 |
| |
|
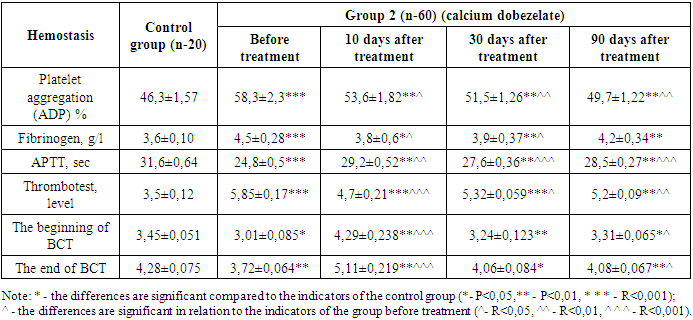
While the coagulogram showed a fibrinogen level of 4,6±0,26 at the beginning of treatment, the fibrinogen content decreased to 3,8±0,04 (P<0,05) by day 10 of treatment, but then increased to 3,9±0,38 (P<0,05) by day 30 and 4,3±0,39 (P<0,01) by 90 days of treatment. Antiplatelet agents do not directly affect fibrinogen, which is considered a criterion of the coagulation hemostasis system. However, anticoagulants recommended in accordance with the traditional standard of treatment at the beginning led to a decrease in this indicator for the first 10 days.Partial activation time (APTT) of thromboplastin also showed positive changes during treatment. On the first day of treatment, the APTT was 24,5±0,6. Against the background of antiplatelet therapy, this indicator was 29,1±0,55 (P<0,001) on the 10th day of treatment, while after 30 days of treatment, a positive change was observed to 27,8±0,32 (P<0,001), and after 90 days - to 28,3±0,28 (P<0,01).When conducting a blood clot test on the first day of treatment, it was found that it was 5,9±0,16 degrees, and on the 10th day of treatment this indicator decreased to 4,6±0,20 (P<0,001), and on the 30th day there was an improvement in indicators to 5,2±0,06 (P<0,05), and by the 90th day of treatment, this indicator decreased to 5,1±0,08 (P<0,01).Process-specific changes were also observed during blood clotting. According to him, on the first day of treatment, the beginning of BCT was 3,02±0,089 minutes, and the end of BCT was 3,76±0,066 minutes. This indicates a significant increase in blood clotting. On the 10th, 30th and 90th days of our study, when BCT was examined, there was a positive change compared to the indicator before treatment. According to him, the beginning of BCT on the 10th day of treatment was 4,32±0,242; the termination of BCT was 5,21±0,228 (P<0,001) minutes, and on the 30th day the beginning of BCT was 3,30±0,126, the end of BCT was 4,08±0,089 (P<0,05) minutes, the beginning of BCT was 3,38±0,067 (P<0,05) minutes after 90 days, and the end of BCT was 4,14±0,0684 (P<0,01) minutes. In patients of the main group, regular intake of antiplatelet drugs led to normalization of BCT indicators, and the process of hypercoagulation was not observed. The use of doxichem in the study gave results below those of the hemostasis system in the recommended patients of the group 2.Prior to treatment, platelet aggregation activity was 58,3±2,3 (P<0,001). After 10 days of treatment with antiplatelet agents, there was a decrease in platelet aggregation activity to 53,6±1,82 (p<0,05). By the 30th day of treatment, this indicator continued to decrease to 51,5±1,26 (P<0,01), and by the 90th day - to 49,7±1,22 (P<0,01). During treatment after 10 days, platelet aggregation decreased by 8,06% compared to day 1, after 30 days - by 11,7% and after 90 days - by 14,8% with a positive change in the improvement of rheological properties of blood (table 3).Table 3. Dynamics of changes in hemostasis indicators during treatment with antiplatelet agents in group 2 patients
 |
| |
|
While the coagulogram index was 4,5±0,28 at the beginning of treatment, the fibrinogen content decreased to 3,8±0,6 (P<0,01) by the 10th day of treatment, but then increased to 3,9±0,37 (P<0,01) on the 30th day and 4,3±0,34 (P<0,05) by the 90th day of treatment. Antiplatelet agents do not directly affect fibrinogen, which is indicated as a criterion of the coagulation hemostasis system. However, anticoagulants recommended in accordance with the traditional standard of treatment at the beginning of treatment led to a decrease in this indicator for the first 10 days. But the mild effect of the anticoagulant in the drug Doxychem was manifested in the fact that it kept fibrinogen at much lower levels compared to the group 2, which received dipiradamole.Changes in the positive side during treatment were also found in the indications for the use of thromboplastin during partial activation. In the first days of treatment, the APTT was 24,8±0,5. On the background of antiplatelet therapy, on the 10th day of treatment, a positive change in this indicator was observed to 29,2±0,52 (P<0,01) seconds, on the 30th day of treatment to 27,6±0,36 (P<0,001), and on the 90th day to 28,5±0,27 (P<0,001) seconds.The blood clot test on the first day of treatment showed 5,85±0,17 degrees, and on the 10th day of treatment, this indicator showed a positive shift to 4,7±0,21 (P<0,001) and on the 30th day to 5,32±0,059 (P<0,05), by the 90th day of treatment, this indicator decreased up to 5,2±0,09 (P<0,01).Blood clotting time also showed specific changes. According to him, the beginning of BCT at the beginning of treatment was 3,01±0,085 minutes, and the end of BCT was 3,72±0,064 seconds. This indicates that blood clotting had increased more than normal. On the 10th, 30th and 90th days of our study, when BCT was examined, there was a positive change compared to the indicator before treatment. According to him, the beginning of BCT on the 10th day of treatment was 4,29±0,238 (R<0,001); the termination of BCT was 5,11±0,219 (R<0,001) minutes, and on the 30th day, the beginning of BCT was 3,24±0,123 (R<0,01); The termination of BCT was 4,06±0,084 (R<0,05) minutes, and on the 90th day, the beginning of BCT was 3,31±0,065 (R<0,05) minutes, the termination of BCT was 4,08±0,067 (R<0,05) minutes. In patients of the main group, regular intake of antiplatelet drugs led to the maintenance of BCT indicators at the regulatory level, and the process of hypercoagulation was not observed.In both groups, in our studies, we observed that changes in the parameters of the hemostasis system change (platelet aggregation activity, fibrinogen, thromboplastin partial activation time, blood clot test, BCT) significantly in a positive direction in relation to the physiological norm. The results obtained in the two groups were processed in a situation that was compared with each other. Looking back at the data, the images showed that platelet aggregation activity in group 1 patients decreased by 8,2% at 10 days, by 11,8% at 30 days, and by 15,02% at 90 days, while in patients of the group 2 in 10 days platelet aggregation decreased by 8,06%, in 30 days it decreased by 11,7%, and in patients in 90 days there was a decrease of 14,8% in the positive indicator of re-improvement of blood parameters. The dynamics reflected an almost identical result in both groups. However, there is an advantage, even though insignificant, in groups taking pentoxifylline.In the studies conducted in groups of patients treated with doxychem for three months, it was clear that the positive effect of the drug on the hemostasis system does not lag behind those who received dipirdamole, and a number of laboratory taxa are based on reliable indicators, such as platelet aggregation, fibrinogen, APTT, testing for blood clots and BCT indicators. In addition, in patients, we studied violations of the filtration function of the kidneys and vascular resistance in patients, in connection with this, the group 1 on the first day of treatment, the creatinine content was 169,4±12,41 µmol/l, 10 days after treatment, creatinine decreased to 164,1±10,72 µmol/l (P<0,05). 30 days after the start of treatment, the content of creatine in the blood was 156,6±7,05 µmol/l (p<0,05) by the 90th day there was a decrease in creatinine to 151,4±7,22 µmol/l (p<0,05).Table 4. Results of laboratory studies of group I patients
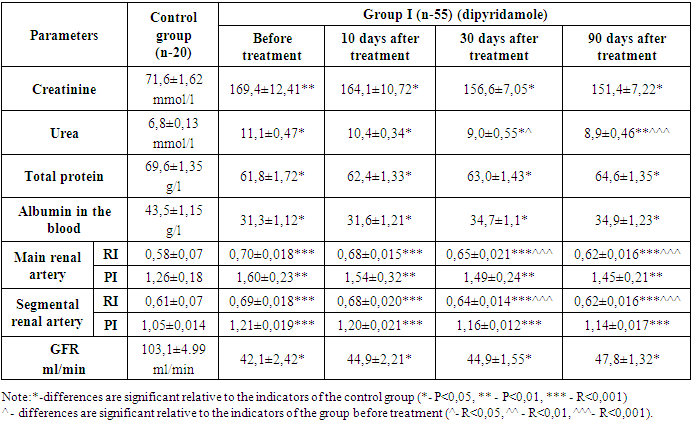 |
| |
|
The urea content in the blood was 11,1±0,47 mmol/l before the start of treatment and decreased to 10,4±0,34 mmol/l (P<0,05). However, by 30 days of treatment, there was a decrease of 9,0±1,55 mmol/l (P<0,05) to 8,9±0,46 mmol/l (P<0,001) for 90 days.The glomerular filtration rate (GFR) was increased from 42,1±2,42 ml/min to 44,9±2,21 ml/min (P<0,05) after treatment. There was an increase in the treatment rate from 44,9±1,55 ml/min for 30 days (P<0,05) to 47,8±1,32 ml/min for 90 days (P<0,05).Thus, patients had increased oncotic plasma pressure due to an increase in the content of albumin in the blood and a parallel total protein. As a result, there was an increase in GFR due to the addition of reserve glomeruli to work activity, and, due to this, a decrease in products such as urea and creatinine in the blood.In the primary and segmental renal arteries, resistance and pulse indices were studied, which determine the level of renal vascular resistance. The resistance index in the main renal artery decreased to 0,68±0,015 (P<0,001) after treatment, with a value of 0,70±0,018 at the beginning of treatment. After 30 days of treatment, this indicator decreased to 0,62±0,016 (P<0,001) by 90 days, which indicates a value of 0,65±0,021 (p<0.001). There was a decrease in the pulse index by 1,54±0,32 (P<0,01). After 90 days of treatment, it was noticed that this indicator decreased to 1,45±0,21 (P<0,01).The resistance index in segmental renal arteries was 0,69±0,018 values at the beginning of treatment - it decreased to 0,68±0,020 (P<0,001) after treatment. After 30 days of treatment, this indicator decreased by 0,62±0,016, and after 90 days, this value was 0,64±0,014 (P<0,001). The above indicators for the second group have changed in this order: Table 5. Results of laboratory studies of group II patients
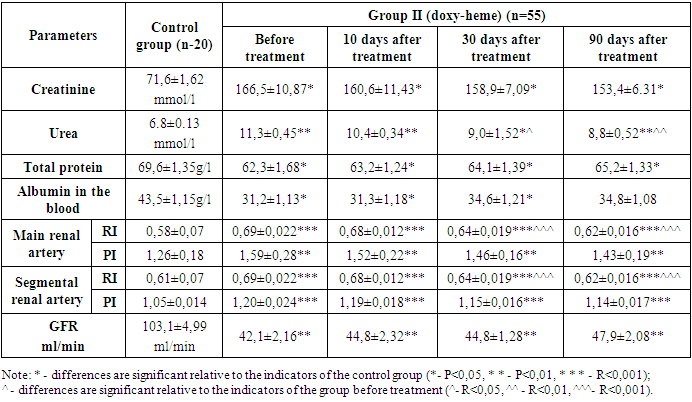 |
| |
|
Before treatment, the urea content in the blood was 11,3±0,45 mmol/l, and after treatment, its amount decreased to 10,4±0,34 mmol/l (P<0,05), by the 60th day it was 9,0±1,52 mmol/l (P<0,05), and after 90 days there was a decrease in the urea level of 8,8±0,52 mmol/l (P<0,01).GFR increased from 42,1±2,16 ml/min to 44,8±2,32 ml/min (P<0,01). However, by the 60th day of treatment, there was an increase in GFR from 44,8±1,28 ml/min (P<0,01) to 47,9±2,08 ml/min (P<0,001).While the total protein was 62,3±1,68 g/l at the beginning of the study, its amount increased to 63,2±1,24 g/l (P<0,05) after treatment, the total protein was 64,1±1,39 g/l (P<0,05), by the 90th day increased to 65,2±1,33 g/l (P<0,05) after the introduction of the protein load. While the albumin content in the blood.According to the results of dopplerography, the resistance index in the main renal artery before treatment was 0,69±0,022 values, after treatment it decreased to 0,68±0,012 (P<0,001). After 30 days, this indicator showed a decrease of 0,62±0,016 (P<0,001), on the 90th day, it showed a value of 0,64±0,019 (p<0,001). There was a decrease in the pulse index by 1,52±0,22 (P<0,01) after treatment compared with the value of 1,59±0,28 before treatment. After 30 days of treatment, it was noted that this indicator decreased by 1,43±0,19 in 90 days (P<0,01), which indicates a value of 1,46±0,16 (P<0,01).The resistance index in segmental renal arteries decreased to 0,68±0,012 (P<0,001) after 10 days with a value of 0,69±0,022 at the beginning of treatment. After 30 days of treatment, this indicator decreased from 0,64±0,019 (P<0,001) to 0,62±0,016 (P<0,001) in 90 days, indicating a value of 0,64±0,019 (p<0,001). The pulse index was 1,20±0,024 before the start of treatment and, as noted, decreased to 1,1±0,018 (P<0,001) after the end of treatment. After 30 days of treatment, it was observed that this indicator decreased by 1,14±0,017 (P<0,001) for 90 days, indicating a value of 1,15±0,016 (p<0,001). We explain that this shift is due to a decrease in the aggregation of shaped elements over several months in patients treated with doxychem for three months.
5. Conclusions
Studying the effectiveness of antiplatelet therapy in the treatment of patients with chronic kidney disease, the study presented the following conclusions:1. In stage II-III SBC, the indicators of the hemostasis system are significantly shifted towards negative, and in patients they begin to exceed the indicators of uremia, such as urea and creatinine, although clinical symptoms are not pronounced, and GFR decreases by about 50%. It is obvious that the positive effect of the drug on the hemostasis system in patients receiving Calcium dobesilate does not lag behind the groups receiving dipyridamole, based on reliable indicators such as platelet aggregation, fibrinogen, APTT (sec), blood clotting time.2. The recommendation of the drug Calcium dobesilate to patients who have SBC for a long time leads to an improvement in the functional state of the kidneys. This was manifested by a decrease in the level of urea and creatinine in the blood and increasing of GFR.
References
| [1] | Smirnov A.V., Shilov E.M., Dobronravov V.A. et al. National recommendations. Chronic kidney disease: basic principles of screening, diagnosis, prevention and treatment approaches. “Lefty. St. Petersburg”, 2012. 51 p. |
| [2] | Am. J. Kidney Dis. 2002., Rebollo-Rubio A. 2015, Kalyujin V.V., 2015. Volume 4. P.87 |
| [3] | Daminova L.T., Shukurova L.Kh., Analysis of the functional state of the kidney and changes in blood rheology in the II-III stage of chronic kidney disease. Scientific and practical magazine “Infection, immunity and pharmacology” No. 6 2022. |
| [4] | Smirnov A.V. Epidemiology and socio-economic aspects of chronic kidney disease / A.V. Smirnov, V.A. Dobronravov, I.G. Kayukov [et al.] // Nephrology. 2006. Vol. 10, No. 1. Pp. 7-13. |
| [5] | Sigitova O.N. Chronic kidney disease and chronic renal failure: modern approaches to terminology, classification and diagnosis. Journal of modern clinical medicine. 2008 Volume 1. Issue 1. Pp. 83-88. |
| [6] | Jha V., Garcia-Garcia G., Iseki K. et al. Chronic kidney disease: global dimension and perspectives. Lancet. 2013; 382: 260–272. |
| [7] | Garcia Garcia G., Harden P.N., Chapman J.R. World Kidney Day 2012. The Global role of kidney transplantation. The Lancet 2012; 379: e36-e38. |
| [8] | Schoolweth A.C. et al., Chronic kidney Disease: A Public Health Problem That Needs a Public Health Action Plan, Preventing Chronic Plan, Preventing chronic Disease-Public Health Research, Practice, and Policy (2006), 3(2) 1–6. |
| [9] | Melnik A.A., The hemostasis system and its regulation in cases of impaired renal function. “Kidneys” 2016 pp. 67-70. |
| [10] | Rebrov A. P., Zelepukina N. Yu. // Nephrology and dialysis. 2001. No. 4. (available by http://www.nephro.ru/magazine/article.php?id=8838).; N.A. Shmakova. The role of endothelial dysfunction in the defeat of the cardiovascular system in glomerulonephritis in children: Abstract. dis ... cand. medical sciences Tomsk, 2005. 20 p. |
| [11] | Yesayan A.M., Kayukov I.G. // Treatment of chronic renal failure / Edited by S.I. Ryabova. - St. Petersburg, 1997. Pp. 26-35.; Tareeva I.E. // Ter. archive. 1996. No. 6. Pp. 5-10.; Mercier E. et al. // The Hematology Journal 2001. 2. P. 18-25.]. Movchan E.A. [Movchan E.A. // Bulletin of Siberian Medicine, 2008. Appendix 2 pp. 88-96. |
| [12] | In the same time Adams M.J. et al. Adams M.J. et al. // Thrombosis Research 2008. V. 123. Issue 2. P. 374-380. |
| [13] | Sardar P. Chatterjee S., Herzog E., etal. Novel oral anticoagulants in patients with renal insufficiency: a meta-analysis of randomized trials. Can J Cardiol 2014; 30(8): 888-97. |
| [14] | Barnoyev Khabib Bobomurodovich, Shukurova Lobar Khusanovna, Khusankhodjaeva Feruza Tulkunovna ASSESSMENT OF RENAL FUNCTIONAL RESERVE AGAINST THE BACKGROUND OF ANTIPLATELET THERAPY IN THE II-III STAGE OF CHRONIC KIDNEY DISEASE // ORIENSS. 2021. No. 3. URL: |
| [15] | “Possibilities of modern anticoagulant therapy in patients with non-valvular etiology of atrial fibrillation and chronic kidney disease” Irina Sergeevna Daabul, Anastasia Andreevna Sokolova, Dmitry Aleksandrovich Napalkov* First Moscow State Medical University named after I.M. Sechenov, Russia, 119991, Moscow, Trubetskaya str., 8 p. 2 |
| [16] | Natochina N.Yu. // Russian bulletin of perinatology and pediatrics. 1999. No. 6. No. 41-46.vanHylckamaVliegAetal // Blood 2000. 95. P.3678-3682. |
| [17] | Wattanakit K., Cushman M. //CurrOpinPulmMed. 2009. 15(5). P. 408-412. |
| [18] | MahmoodiBK // JAmMedAssoc 2009; 301. P.1790-1797. Kayali F, //Am JMed 2008. 121. P. 226-230. |
| [19] | Keller C et al. // BMCNephrol 2008. 9. P.9. |
| [20] | Shlipak MG et al. //Circulation 2003; 107. P.87-92. |
| [21] | Bach LD. et al. // American Journal of Kidney Diseases 2009. V.53. Issue 4. P. 596-605. |
| [22] | Małyszko J et al. //Kidney & Blood Pressure Research 2004. 27. N 2. P.71-77. |




 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML



