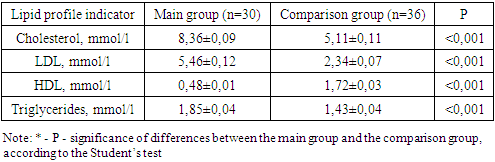-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2023; 13(10): 1477-1481
doi:10.5923/j.ajmms.20231310.25
Received: Aug. 20, 2023; Accepted: Sep. 25, 2023; Published: Oct. 10, 2023

Features of the Clinical Course and Diagnosis of Hyperandrogenia in Mayer-Rokitanski-Kuster-Hauser Syndrome
Adilova M. N.1, Negmadjanov B. B.2, Rabbimova G. T.3
1Applicant of the Department of Obstetrics and Gynecology No. 2, Samarkand Medical University, Uzbekistan
2Doctor of Medical Sciences, Professor, Samarkand Medical University, Uzbekistan
3Candidate of Medical Sciences, Associate Professor, Samarkand Medical University, Uzbekistan
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Congenital utero-vaginal aplasia or aplasia Mayer-Rokitansky-Küster-Hauser syndrome (MRKH) is characterized by unfused uterine buds, aplasia of the cervix and vagina, but normal or hypoplastic bilateral adnexa and is clinically manifested by primary amenorrhea. Patients with MRKH have a normal developmental female phenotype and karyotype (46,XX) and an incidence of 1 in 4000 or 5000 births (Cheroki et al. 2006). MRKH syndrome is considered to be a malformation of the Müllerian (paramesonephric) ducts, occurring in utero between 4 and 12 weeks of gestation, in the oviducts, uterus, cervix and upper vagina, while the Wolffian (mesonephric) ducts regress without fusing with the Müllerian ducts ( Ludwig 1998).
Keywords: Clinical, Course, Mayer-rokitanski
Cite this paper: Adilova M. N., Negmadjanov B. B., Rabbimova G. T., Features of the Clinical Course and Diagnosis of Hyperandrogenia in Mayer-Rokitanski-Kuster-Hauser Syndrome, American Journal of Medicine and Medical Sciences, Vol. 13 No. 10, 2023, pp. 1477-1481. doi: 10.5923/j.ajmms.20231310.25.
1. Introduction
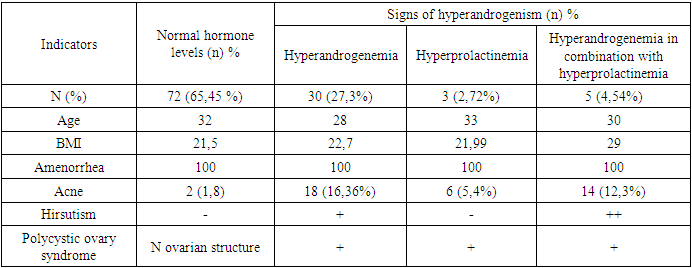
- Mayer-Rokitansky-Küster-Hauser syndrome (MRKH) is a rare congenital disorder of the female reproductive system that is characterized by the absence or underdevelopment of the uterus and vagina. However, women with MRKH may experience hyperandrogenism, which is associated with increased levels of male hormones in the blood. Hyperandrogenism (HA), on the other hand, is a condition in which the levels of male hormones such as testosterone, dihydrotestosterone and dehydroepiandrosterone (DHEA) are elevated in a woman. According to the literature, 10–20% of women exhibit certain signs; HA is not only a medical, but also a social problem, since it leads to various cosmetic defects (excessive oily skin and hair, the development of acne), as well as psycho-emotional reactions that reduce the quality of life of women, as well as social problems associated with restrictions in choice professions and employment. In women with MRKH, hyperandrogenism may occur due to dysfunction of the ovaries, which continue to secrete male hormones into the blood despite the absence of the uterus and vagina. Symptoms of hyperandrogenism may include a rough voice, increased muscle mass, increased hair growth on the face, chest, and abdomen, and menstrual irregularities. In some cases, hyperandrogenism can lead to impaired fertility, as well as an increased risk of developing cardiovascular disease and diabetes. To diagnose hyperandrogenism in patients with MRKH, it is necessary to conduct a comprehensive examination, including analysis of the level of male hormones in the blood, assessment of the clinical manifestations of hyperandrogenism, ultrasound examination of the ovaries, assessment of insulin and blood sugar levels, as well as genetic studies. Treatment of hyperandrogenism in MRKH syndrome may include the use of hormonal medications, such as contraceptives or androgen antagonists, which reduce the level of male hormones in the blood and improve the clinical symptoms of hyperandrogenism. There is little literary data on the endocrinological features of the condition of the ovaries with aplasia of the uterus and vagina, and they are contradictory. There are isolated publications on data on hyperandrogenism syndrome in patients with aplasia of the vagina and uterus. Therefore, the study of patients with aplasia of the vagina and uterus in combination with hyperandrogenism of ovarian origin, the characteristics of clinical and diagnostic criteria are relevant.The purpose of the study was to study the features of the clinical course and diagnosis of hyperandrogenism syndrome with aplasia of the vagina and uterus.
2. Material and Research Methods
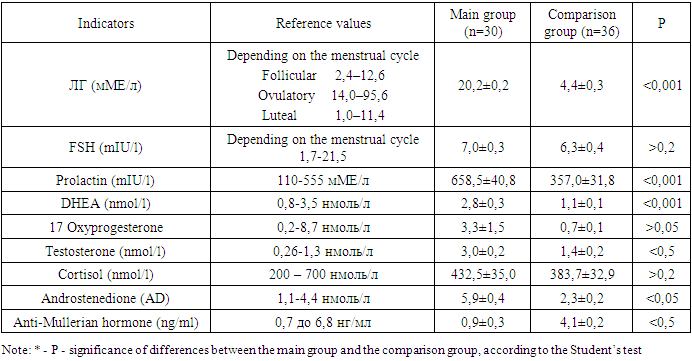
- The studies were carried out in the department of pediatric gynecology of a multidisciplinary medical center and the private company “Doctor” in the period from 2009-2023. Clinical data and results of diagnostic criteria of 110 patients with Rokitansky-Küster-Hauser-Mayer syndrome were studied. Criteria for inclusion in the main group: patients with aplasia of the vagina and uterus in combination with one or more clinical (acne, alopecia, hirsutism) manifestations of hyperandrogenism (clinical and laboratory signs) aged 16-35 years; Exclusion criteria: patients with aplasia of the vagina and uterus aged less than 16 and more than 35 years; androgen-producing tumors, hypothyroidism, type 1 diabetes mellitus, hyperprolactinemia of organic origin, hypercortisolism, taking medications with side effects of hyperandrogenism. Determination of hormone levels was carried out in all patients at the first visit and over time using enzyme immunoassay. Hormones such as testosterone, TSH, LH, FSH, DHEAS, and prolactin were determined using an enzyme immunoassay in the laboratory. Assessment of the severity of acne and hirsutism in hyperandrogenism of ovarian origin can be carried out using various scales and indices. One of the most common indices of hirsutism is the Ferriman-Gallwey index, which evaluates the level of hairiness on the face, chest, abdomen, back and upper extremities on a 9-point scale. According to the Acne Qualification Group scale, the severity of acne was assessed based on the number of inflammatory elements on the face, as well as the number of comedones.
3. Research Results
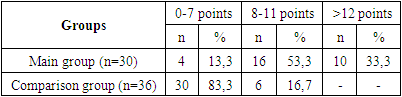
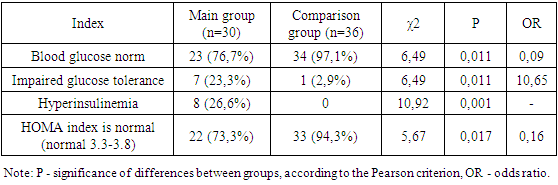
- We assessed the severity of acne and hirsutism in patients of the study groups. There is an opinion that the clinical symptoms of hyperandrogenism develop in direct dependence on the degree of androgen metabolism disorders. When assessing the severity of acne, the most commonly used classification of the American Academy of Dermatology was used: grade I is characterized by the presence of comedones and up to 10 papules; II degree – comedones, papules, up to 10 pustules; III degree – comedones, papules, pustules, up to 5 nodes; IV degree is characterized by a pronounced inflammatory reaction in the deep layers with the formation of painful nodes and cysts. When examining patients, 30.8% of subjects were diagnosed with acne of varying severity. When assessing the severity of acne, the most commonly used classification of the American Academy of Dermatology was used: grade I is characterized by the presence of comedones and up to 10 papules; II degree – comedones, papules, up to 10 pustules; III degree – comedones, papules, pustules, up to 5 nodes; IV degree is characterized by a pronounced inflammatory reaction in the deep layers with the formation of painful nodes and cysts. Based on the accepted classification, the vast majority of patients in the main clinical group had grade I acne in 46.6% of cases, and grade II acne was noted in 25.6% of patients. Grades III and IV (the most severe) were recorded in 11.5% and 16.2% of cases, respectively. Despite the lack of a clear relationship between the severity of acne and the presence of biochemical markers of hyperandrogenism, it should be noted that the subjects most often had II and III severity of acne (p = 0.001). In the control group, there were no cases of acne in accordance with the accepted classification. The most significant symptom in the diagnosis of hyperandrogenism is hirsutism. In contrast, acne or seborrhea is more often the result of changes in androgen metabolism. In this regard, in all patients, the hirsute number was determined by the sum of the indifferent (the degree of hair growth of the forearms and legs) and hormonal numbers (the degree of hair growth of the remaining 9 areas of the body), using the classic 4-point Ferriman-Gallwey scale by D. Ferriman and J. Gallwey [3].The total number of points (hirsut number) from 1 to 7 characterizes normal hair growth, from 8 to 11 points – borderline, more than 12 points – hirsutism. Hair growth is considered excessive when the hirsute number is more than 12 points. In the control group, hirsutism was not detected in any case. In the main group, a hirsute number of more than 12 points on the Ferriman-Gallway scale was detected in 33.3%, in 53.3% of cases the hirsute number was within the border zone from 8 to 11 points (Table 1).
|
|
|
|
|
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML