-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2023; 13(10): 1448-1455
doi:10.5923/j.ajmms.20231310.19
Received: Sep. 10, 2023; Accepted: Sep. 26, 2023; Published: Oct. 8, 2023

Development of a Scale of Severity of Chronic Heart Failure in Women with Breast Cancer
Abdullaev Timur Atanazarovich , Alieva Zukhra Khamitovna , Soy Igor Arsenevich
Republican Specialized Scientific and Practical Medical Center of Cardiology of the Ministry of Health of the Republic of Uzbekistan, Tashkent, Uzbekistan
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

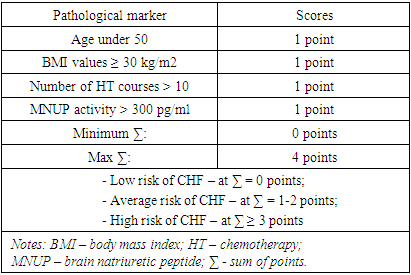
Objective: To study the effects of age, body mass index, chemotherapy and brain natriuretic peptide activity in a comprehensive assessment of the severity of chronic heart failure. Material and methods: 49 women suffering from breast cancer were examined, the average age of which was 55.1±8.7 years. Women over 70 years of age were not included in this fragment of the study. All patients suffered from breast cancer and CHF. All women included in the study, in addition to collecting and analyzing anamnestic data, conducted general clinical and laboratory studies, calculated body mass index (BMI). The severity of CHF was assessed by determining the functional class (FC II-III according to NYHA). Results: The category of women 50+ was characterized by an increased level of indicators of central hemodynamics, which was probably due to the prevalence of such cardiovascular pathologies as coronary heart disease and GB. On the contrary, the presence of DM was more often registered in women younger than 50 years, which, in turn, in combination with the BMI indicator (namely, the predominance of the number of patients with a BMI level > 30 kg/m2), indicates metabolic disorders at a younger age. In women with breast cancer, despite their young age, the absence of obvious signs of coronary heart disease and a low percentage of GB (20.0%), even with preserved LV volume indicators, there was a more pronounced tendency to develop heart failure with the formation of an eccentric type of remodeling of the heart muscle, which emphasizes / proves a more malignant (from the standpoint of the development of CVD) course of the disease in women younger than 50 years. Despite the absence of intergroup (depending on age) differences in the level of MNUP activity (all p > 0.05), nevertheless, the correlation analysis showed that in women with breast cancer, as the MNUP grew, there was a decrease in LVL, which makes it possible to identify MNUP as an marker in the development of CHF in patients suffering from breast cancer. Age younger than 50 years; BMI values ≥ 30 kg/m2; the number of CT courses ≥ 10 and MNUP activity > 300 pg/ml – can be considered as markers in assessing the risk of developing and severity of CHF in patients with breast cancer. Conclusion: Our study shows that in women suffering from breast cancer, it is necessary to conduct a comprehensive assessment of the combination of risk factors such as age, BMI, MNUP activity and the number of CT courses.
Keywords: Breast cancer, Risk factors, Age, Body mass index, Chemotherapy, Brain natriuretic peptide, Chronic heart failure
Cite this paper: Abdullaev Timur Atanazarovich , Alieva Zukhra Khamitovna , Soy Igor Arsenevich , Development of a Scale of Severity of Chronic Heart Failure in Women with Breast Cancer, American Journal of Medicine and Medical Sciences, Vol. 13 No. 10, 2023, pp. 1448-1455. doi: 10.5923/j.ajmms.20231310.19.
Article Outline
1. Relevance
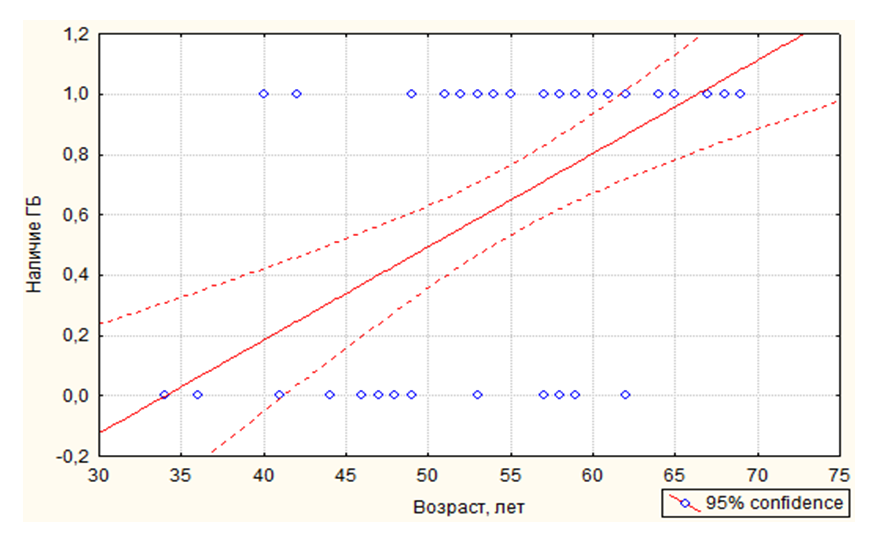
- Patients with breast cancer (breast cancer) may have a higher risk of cardiovascular disease (CVD) compared to the general population. Although modern cancer treatment methods, in particular, breast cancer (schemes based on anthracyclines, trastuzumab and radiation therapy (LT)), significantly reduce the risk of cancer recurrence and death, nevertheless they are associated with an increased risk of CVD [1-3]. According to literature data, chemotherapy (CT) based on anthracyclines and trastuzumab increases the risk of heart failure (HF) by 5 times compared to regimens without these components [4].Another reason why patients with breast cancer may have a higher risk of CVD is that the risk factors (FR) of both diseases overlap, especially such FR as obesity and inactivity [5]. Patients with breast cancer may have a higher prevalence of FR CVD than in the general population. Pre-existing FR CVD was also associated with a higher risk of cardiotoxicity caused by cancer treatment [6-7]. Despite all the complexity and the areas being studied, the problem of women suffering from breast cancer in the aspect of the development of chronic heart failure (CHF), its early diagnosis and assessment of its severity, especially taking into account the cardiotoxicity of CT, is a very relevant and in-demand problem of modern cardio-oncology. In addition, due to the improvement of diagnostics in recent decades, there has been a rejuvenation of breast cancer. However, the question of the influence of age on the prognosis for breast cancer remains controversial. Some authors claim [8] that survival rates in breast cancer are comparatively lower for women under 40 than for older women, regardless of the histological type of and the stage of the process. Others write that breast cancer is the most common cancer among pregnant and lactating women and occurs in one case for every 3000-6000 pregnancies [9]. Nevertheless, in the literature we analyzed, we did not find data on the assessment of the influence of age on the severity of CHF in women suffering from breast cancer, which was the purpose of the presented scientific work.
2. Material and Methods
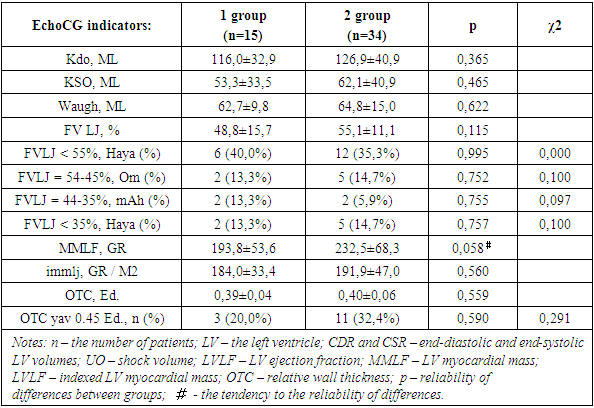
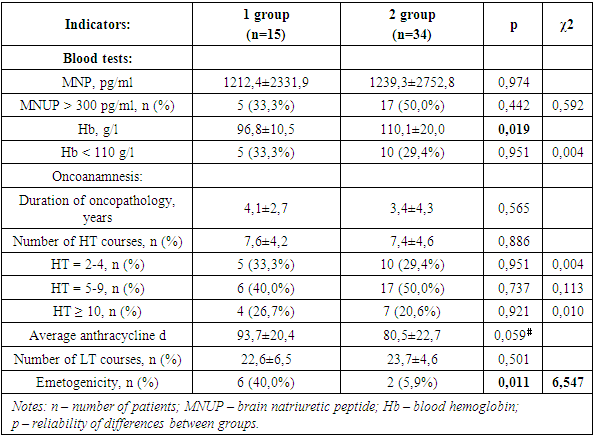
- 49 women suffering from breast cancer were examined, the average age of which was 55.1±8.7 years. Women over 70 years of age were not included in this fragment of the study. All patients suffered from breast cancer and CHF. All women included in the study, in addition to collecting and analyzing anamnestic data, conducted general clinical and laboratory studies, calculated body mass index (BMI). The severity of CHF was assessed by determining the functional class (FC II-III according to NYHA).Instrumental studies included the removal and decoding of ECG in 12 standard leads with registration and analysis of cardiac arrhythmias (LDC), as well as EchoCG with the determination of indicators – end-diastolic (CDR, ml), end-systolic (CSR, ml) volumes of the left ventricle (LV), stroke volume (UO, ml) and left ventricular ejection fraction (LVLF).LV myocardial mass (MMLH) was calculated using the "area – length" method, the results obtained were indexed in relation to the body surface area (the so-called indexed myocardial mass - IMLH). As the upper bound of the IMLF, a value for women of 104 g/cm2 was used, according to De Simone. The index of the relative wall thickness (OTC) in the diastole was calculated by the formula:OTC = (TMJP + TSSLJ) / CDR,where TMJP is the thickness of the interventricular septum, TSSLJ is the thickness of the posterior wall of the LV, CDR is the finite diastolic size of the LV. All indicators were calculated in cm. Based on the values of IMLJ and OTS, the following geometric types of LV were distinguished [7,8]: – normal geometry (IMLJ≤N, OTS<0.45); – concentric remodeling (IMLJ≤N, OTC≥0.45); – concentric hypertrophy (IMLJ>N, OTC≥0.45); – eccentric hypertrophy (IMLJ>N, OTC<0.45).According to the results of the 6-minute walk test (TSHX), the functional class (FC) of CHF was determined by measuring the length of the distance traveled (DPD, m), as well as by the SHOCK scale.
3. Statistical Analysis
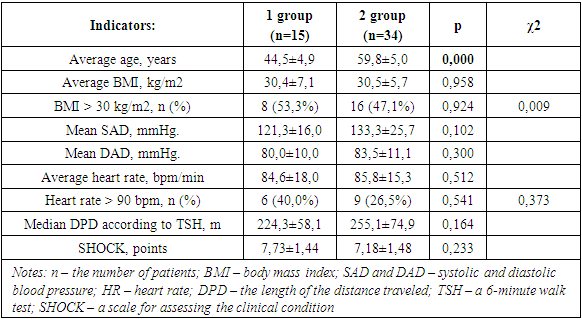
- The data were described as mean ± standard deviation (M±SD) for the interval and quantity (%) for categorical variables. We used the chi-square criterion and the Fisher criterion for categorical variables. The Student's criterion was used for numerical variables. To assess the presence of links between the indicators, a correlation analysis was carried out with the calculation of the Pearson correlation coefficient. The value of p ≤ 0.05 was considered a statistically significant result.Depending on the age, two groups were identified: group 1 – 15 women aged 30-50 years and group 2 – 34 women aged 51-70 years (since the patients were female, the premenopausal and menopausal periods served as the age selection category).
4. The Results of the Study
- In the course of the study, it was found that, despite the different age categories, women of both the 1st and 2nd groups had comparable BMI values (Table.1). This was confirmed by the correlation analysis, which did not reveal any relationship between the age of women and BMI values (p=0.976; r=0.004; t=0.030). Nevertheless, the number of women with a BMI > 30 kg/m2 (i.e. with obesity) prevailed by 6.2% among group 1 individuals.
|
|
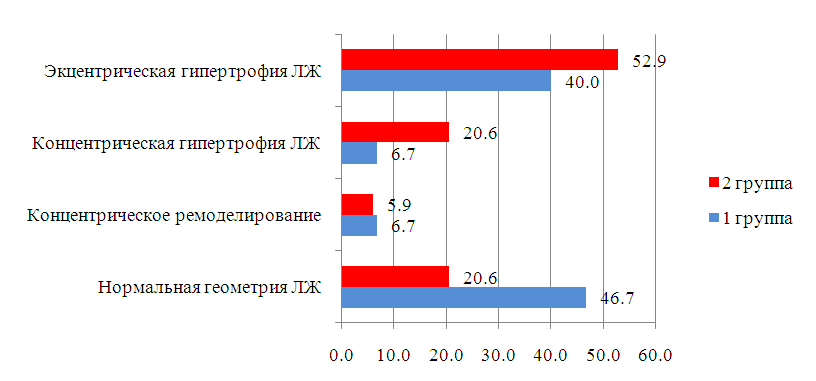 | Figure 3. Geometric types of the left ventricle in the compared groups of women. (Notes: The data are presented as a percentage; LV – left ventricle.) |
|
|
5. Discussion
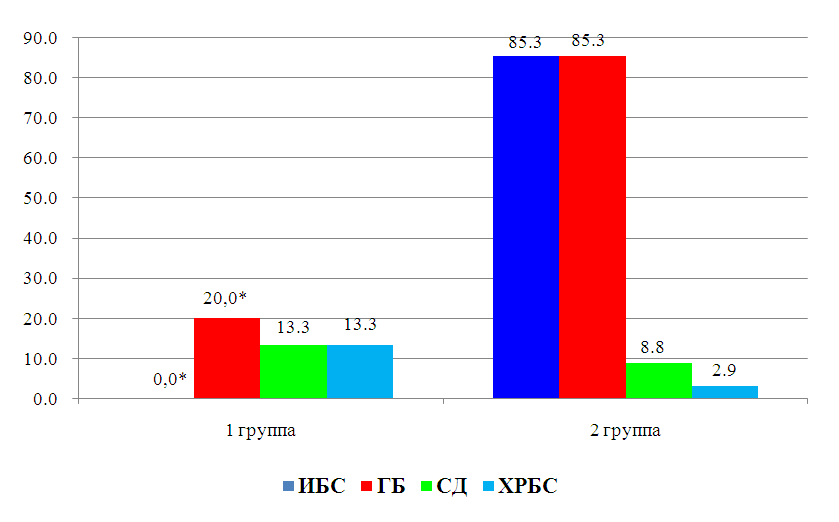
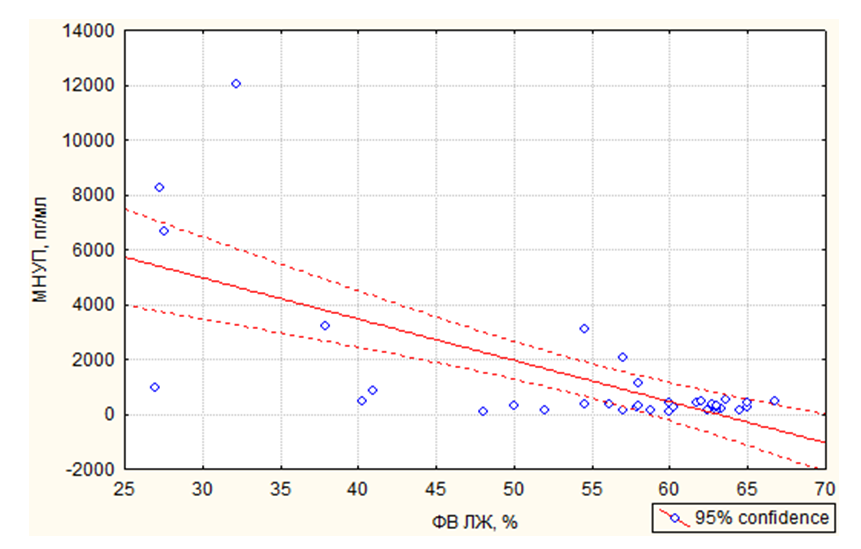
- Age, reproductive factors, personal or family history of breast diseases, genetic predisposition and environmental factors are associated with an increased risk of developing breast cancer. The peak incidence of breast cancer occurs during the postmenopausal period and accounts for up to 75% of all cases. On the contrary, in women younger than 35 years, the incidence of breast cancer is low – less than 5% of all cases [10]. This was confirmed in our study, where it was also revealed that among patients suffering from breast cancer, the number of women over 50 years old was more than 2 times more than the number of young women.According to literature data, with obesity in women, changes in the function of the hypothalamic-pituitary system occur, hypothalamic control over the production of follicle-stimulating and luteinizing hormones of the ovary is disrupted. These disorders lead to amenorrhea, menorrhagia or prolongation of the menstrual cycle – which are risk factors for breast cancer [11]. In our study, the average BMI, regardless of age, was about 30.5 kg/m2, but the number of women with a BMI level > 30 kg/m2 among young women was 6.2% more than among women over 50 years old. In addition, women under the age of 50 had more cases of DM and HRBS, which, being systemic, could indirectly affect the hormonal background of our patients. It is widely known that obesity is almost always combined with diabetes and hypertension. Studies show that the combination of diabetes and heart disease increases the risk of developing breast cancer by 2.2 times [12]. This is also in tune with our data. In particular, in the women we examined, the presence of GB occurred in 85.3% of cases among women over 50 years old and in 20.0% of cases in young patients, among whom, as mentioned above, DM was more often registered (13.3% in group 1 versus 8.8% in group 2) and BMI > 30 kg/m2. Cardiotoxicity of chemotherapeutic drugs has a wide range of clinical manifestations: LV diastolic dysfunction (DD), HF, hypertension, vasospastic and thromboembolic ischemia, as well as various LDC [13-14]. Despite the variety of chemotherapeutic agents, anthracycline drugs remain the most commonly used in the treatment of breast cancer and are widely studied. Their use is associated with various mechanisms of cardiotoxicity development [15-16]. In relation to our study, this fact manifested itself as a decrease in the LVL index with the formation of an eccentric type of remodeling. According to the literature data, the cardiotoxic effect of CT is manifested by symptoms such as systolic or diastolic ventricular dysfunction with the development of dilated cardiomyopathy and a decrease in LV mass and wall, as well as possible cardiac rhythm and conduction disturbances, and a decrease in contractility [17], which also occurred in our patients.Back in the 90s of the last century, the brain natriuretic peptide (MNUP), the most important peptide, was considered as the "Gold standard" for the prediction of CHF. The ventricles of the heart secrete it during overloads. Its secretion reflects changes in hemodynamics and signals LV dysfunction. An increase in its level indicates CH [18]. In our study, the activity of MNUP was more pronounced in women over 50 years of age. In general, according to the sample, an increase in MNUP was reliably associated with a decrease in LVL, which allows us to assert that it is informative in assessing the severity of CHF even in patients with breast cancer and to single it out as an unfavorable marker.Thus, our study shows that in women suffering from breast cancer, when assessing the risk of developing CHF, it is necessary to pay close attention to the study of age-related features and a combination of risk factors such as BMI, MNUP activity and the number of CT courses.
6. Conclusions
- The category of women "50+" was characterized by an increased level of indicators of central hemodynamics, which was probably due to the prevalence of such cardiovascular pathologies as coronary heart disease and GB. On the contrary, the presence of DM was more often registered in women younger than 50 years, which, in turn, in combination with the BMI indicator (namely, the predominance of the number of patients with a BMI level > 30 kg/m2), indicates metabolic disorders at a younger age.In women with breast cancer, despite their young age, the absence of obvious signs of coronary heart disease and a low percentage of GB (20.0%), even with preserved LV volume indicators, there was a more pronounced tendency to develop heart failure with the formation of an eccentric type of remodeling of the heart muscle, which emphasizes / proves a more malignant (from the standpoint of the development of CVD) course of the disease in women younger than 50 years.Despite the absence of intergroup (depending on age) differences in the level of MNUP activity (all p > 0.05), nevertheless, the correlation analysis showed that in women with breast cancer, as the MNUP grew, there was a decrease in LVL, which makes it possible to identify MNUP as an unfavorable marker in the development of CHF in patients suffering from breast cancer.Age younger than 50 years; BMI values ≥ 30 kg/m2; the number of CT courses ≥ 10 and MNUP activity > 300 pg/ml – can be considered as unfavorable markers in assessing the risk of developing and severity of CHF in patients with breast cancer.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML