-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2023; 13(10): 1417-1422
doi:10.5923/j.ajmms.20231310.12

Methods of Early Diagnosis of Ankylosing Spondyloarthritis Based on Clinical-Immunogenetic Indications
Saidrasulova Gulizebo1, Mirakhmedova Khilola1, Dadabayeva Neilya1, Annaev Muzaffar2
1Department of Propaedeutics of Internal Disease No 1, Tashkent Medical Academy, Tashkent, Uzbekistan
2Department of Internal Medicine No 2 and Cardiology, Samarkand State Medical University, Uzbekistan
Copyright © 2023 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Ankylosing spondylitis (AS) is a rheumatologic disease predominantly affecting the sacroiliac joints and axial skeleton, with a notable male predominance and familial inheritance. Inflammatory processes in AS are associated with proinflammatory cytokines, including tumor necrosis factor-alpha (TNF-α), interleukin-17 (IL-17), and gamma-interferons. This disease also involves peripheral joints and extra-axial involvement. Matrix metalloproteinases (MMPs) play a crucial role in extracellular matrix degradation and have been linked to joint erosions in arthritis. In AS, bone remodelling processes are significant, with transforming growth factor-beta 1 (TGF-β1) playing a role in bone repair and immune regulation. This article investigates early diagnostic markers, radiological progression, and clinical immunogenetic factors in AS patients. A study conducted on 100 AS patients revealed a correlation between disease duration and the frequency of uveitis and psoriasis. The presence of the HLA-B27 gene was associated with a higher occurrence of uveitis, especially in patients with longer disease duration. Structural progression, assessed by the modified Stoke Ankylosing Spondylitis Spinal Score (mSASSS), was influenced by disease duration, suggesting its value as an indicator of disease progression. Elevated levels of MMP-3, which are significantly higher in patients with more frequent erosions, highlight its potential as a marker for structural progression. Furthermore, elevated IL-17 levels were observed in AS patients, particularly in the established and late stages, indicating its role in promoting structural changes in the spine and sacroiliac joints. A strong positive correlation between IL-17 and MMP-3 levels underscores their interconnected role in AS pathogenesis. Additionally, increased TGF-β1 levels suggest osteoblast activation in AS patients. These findings shed light on AS pathogenesis and progression, emphasizing the potential of IL-17 and MMP-3 as valuable markers for assessing disease activity and structural changes. These insights may have implications for the development of targeted therapeutic interventions in AS.
Keywords: Ankylosing spondylitis, Cytokines, Matrix metalloproteinases, TGF-β1, IL-17, HLA-B27
Cite this paper: Saidrasulova Gulizebo, Mirakhmedova Khilola, Dadabayeva Neilya, Annaev Muzaffar, Methods of Early Diagnosis of Ankylosing Spondyloarthritis Based on Clinical-Immunogenetic Indications, American Journal of Medicine and Medical Sciences, Vol. 13 No. 10, 2023, pp. 1417-1422. doi: 10.5923/j.ajmms.20231310.12.
1. Introduction
- Ankylosing spondylitis (AS), which typically debuts around the age of 45, is considered a rheumatologic disease characterized by inflammation primarily affecting the sacroiliac joints and the axial skeleton and is more commonly seen among males. The onset of AS is primarily associated with the inflammation of the entheses, which are fibrous attachments between tendons and bones, located especially in areas such as the spine and pelvis. This inflammation leads to the production of proinflammatory cytokines such as tumor necrosis factor-alpha (TNF-α), interleukin-17 (IL-17), and gamma-interferons [1,2]. AS not only affects the skeletal system but also involves peripheral joints and organs such as the eyes, kidneys, and intestines. Another distinctive feature of the disease is the presence of familial inheritance, indicating a hereditary predisposition among close relatives. Various genetic factors, in addition to the presence of the HLA-B27 gene, contribute to this association.Matrix metalloproteinases (MMPs) are enzymes in the body responsible for cleaving extracellular matrix (ECM) proteins. MMPs are considered one of the main pathological factors involved in the degradation of ECM in diseases such as rheumatoid arthritis. MMPs are produced under the influence of cytokines such as IL-1 and TNF-α from fibroblasts, macrophages, synovial cells, endothelial cells, neutrophils, and chondrocytes. Scientific studies have reported an increase in MMP activity within the joints in arthritis, and the quantity of MMP-3 in the blood has been linked to the frequency of synovitis, which marks the beginning of arthritis [3]. In research conducted among rheumatoid arthritis patients and healthy control groups, it has been observed that the levels of MMP-3 are higher in patients with the disease. When analyzed, a higher level of MMP-3 has been associated with more frequent joint erosions in affected individuals [4,12,13].In AS, bone remodelling processes are prominent [8,9,14]. The body maintains the homeostasis of bones through a constant process of bone fragmentation and regeneration. During bone fragmentation, various signaling molecules are released from the bone mesenchyme and migrate to mesenchymal stem cells (MSCs), contributing to bone repair. One of these molecules is transforming growth factor-beta 1 (TGF-β1). TGF-β1, along with bone morphogens, plays a crucial role in both embryonic bone formation and postnatal bone homeostasis. Initially, TGF-β1 is synthesized as a large molecule and is later cleaved into active TGF-β1 and latent-associated protein (LAP). TGF-β1 remains inactive, associated with LAP, within the bone until bone feeding or fragmentation begins. When bone is damaged or fragmented, TGF-β1 and LAP dissociate, and TGF-β1 becomes an active cytokine. Active TGF-β1 influences chondrocytes to repair damaged bone, and chondrocytes, in turn, activate osteoblast cells. In AS, the initial bone fragmentation process occurs, followed by metaplasia at the site of erosion. Eventually, pathological bone-to-bone transformation or syndesmophytes develop in place of metaplasia. TGF-β1 not only plays a role in bone remodeling but also participates in regulating immune processes. The reduction of this factor in the body may lead to the initiation of immune activation processes, which could potentially trigger inflammation.The pathogenesis of AS has not been fully elucidated and remains complex, with interconnections among various pieces of knowledge. However, recent scientific research has shed light on the role of the cytokine IL-17A in the pathogenesis of this disease. In fact, IL-17A inhibitors, such as secukinumab, are being used to treat AS patients [5,11,15], and there are several ongoing scientific investigations related to their impact. Before the use of IL-17 inhibitors in AS, TNF-α inhibitors were widely employed and continue to be used. There are opposing opinions regarding the effect of TNF-α inhibitors on bone remodeling, specifically on pathological bone growth. On the other hand, there is limited information about the ability of IL-17A inhibitors to halt syndesmophyte formation. IL-17A is believed to exert local effects within the body. When IL-17 inhibitors are used in AS patients, improvements in gut inflammation and uveitis have been observed in certain cases [6,10]. Our research has shown changes in IL-17A levels in different groups at both early and established stages of AS, indicating that more information is needed regarding the impact of IL-17A on new bone formation in AS.One of the distinctive features of AS is the ability to observe structural changes simultaneously with the radiological signs of sacroiliac joint and spinal involvement, along with elevated levels of C-reactive protein (CRP) in patients. Pain intensity in the disease is not always correlated with mediators of inflammation, markers, or radiographic changes [7,8,9]. Until recently, proving laboratory markers that predict structural changes in AS ahead of time has remained elusive.Therefore, this article aims to provide an early diagnosis of AS, its evolving characteristics, radiological progression, and the clinical immunogenetic factors that influence which symptoms will manifest.
2. Materials and Methods
- During the period from 2020 to 2023, a research study was conducted at the Republic Rheumatology Center and the Specialized Outpatient Treatment Course of the Tashkent Medical Academy's Multidisciplinary Clinical Center in 100 patients diagnosed with AS in inpatient and outpatient examinations. Out of the 100 patients, 40 were diagnosed with early-stage AS based on X-ray findings, while the remaining 60 were diagnosed with established and late-stage AS. The diagnosis of patients was confirmed using the 2009 ASAS classification criteria and modified New York classification criteria. In the course of the research, all patients completed a clinical questionnaire. Laboratory tests included a complete blood count, C-reactive protein (CRP) level, HLA-B27 gene analysis, IL-17A level in peripheral blood, MMP-3 level, and TGFB-1 level. IL-17A, MMP-3, and TGFB-1 levels were determined using enzyme-linked immunosorbent assay (ELISA). The reference ranges were as follows: IL-17A ranged from 31.25 to 2000 ng/ml, MMP-3 ranged from 0.41 to 100 ng/mL, and TGFB-1 ranged from 18 to 4000 pg/ml. Instrumental examinations included radiographic and MRI evaluations of the sacroiliac joints and the spine. Additionally, disease activity was assessed using BASDAI, ASDAS-CRP, and ASDAS-ESR indices, and radiographic grading of sacroiliac joints was performed following the Kellgren radiographic classification. The evaluation of spinal mobility and structural changes was performed using the mSASSS index. The scoring was as follows: no change in the spine - 0 points, injury, sclerosis, or squaring - 1 point, ossified syndesmophytes - 2 points, and complete bridging syndesmophyte - 3 points. The total scores were collected, and the mSASSS index was calculated within the range of 0-72. Radiographic assessment of the abovementioned structural changes was conducted in the lumbarand cervical spine lateral views.In the research, patients with severe heart, liver, and kidney diseases, as well as individuals aged 18 or older, participated. Patients taking genetically engineered drugs (infliximab, adalimumab, etanercept, IL-17 inhibitors such as secukinumab, ixekizumab), synthetic disease-modifying antirheumatic drugs (sulfasalazine, methotrexate, leflunomide), or bisphosphonate drugs were excluded from the study.Statistical analysis was performed using Student’s t test, the Mann‒Whitney test, and the Pearson correlation coefficient.The research design schematic is provided below:
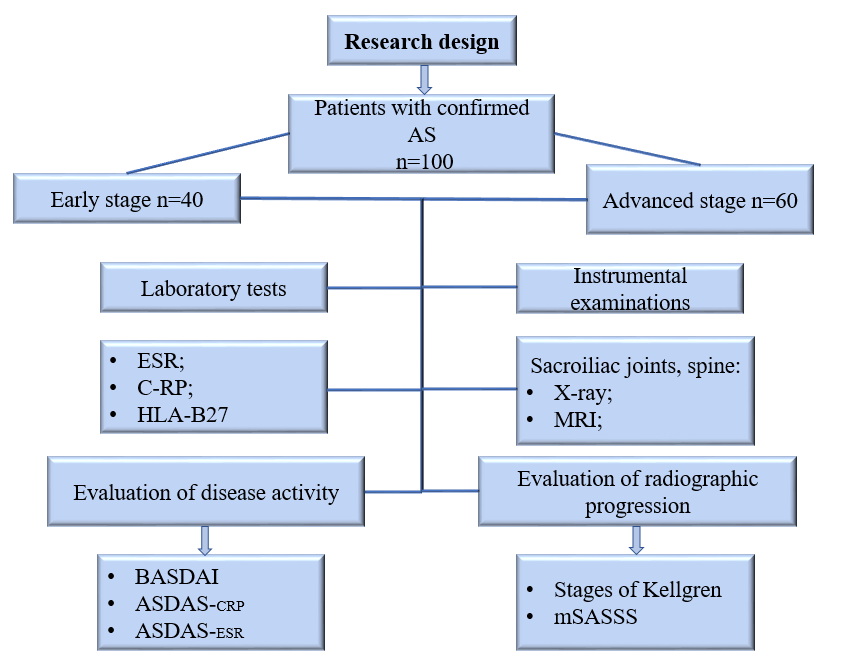 | Figure 1. Research design |
3. Results and Discussion
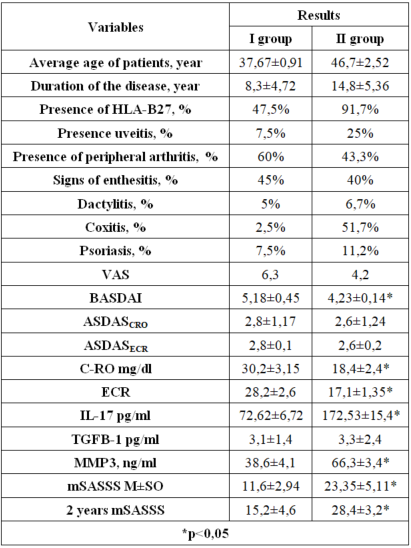
- The participants in the study had an average age of 42.19 ± 1.93 (M± standard deviation), ranging from a minimum of 19 to a maximum of 60 years. The age distribution was as follows: the first group had an age range of 19 to 38, while the second group had an age range of 22 to 60. The average duration of the disease was10.8 ± 7.72 years. However, it is worth noting that in the second group, the disease duration was approximately 15 years on average (Table 1). In the second group, the patients not only had a longer duration of the disease but also tended to be relatively older compared to the first group.
|
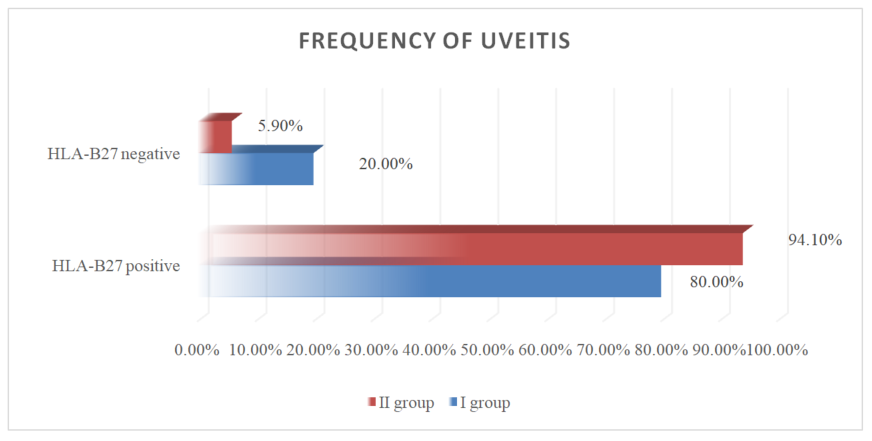 | Figure 2. Frequency of uveitis in two groups of patients with different genetics |
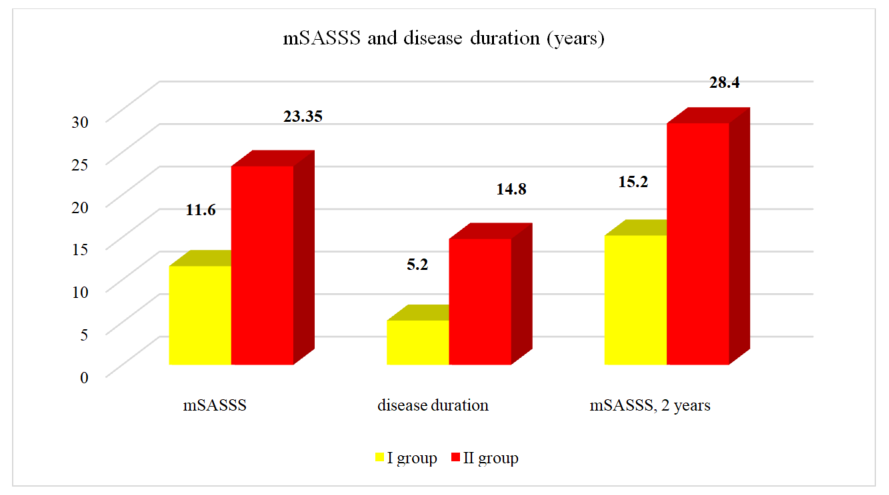 | Figure 3. mSASSS and disease duration (years) in different groups of patients |
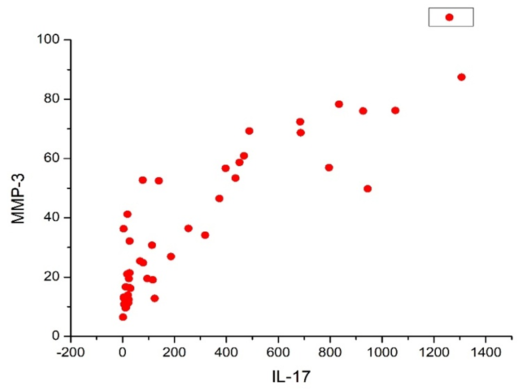 | Figure 4. Correlation between MMP-3 and IL-17 |
4. Conclusions
- In conclusion, the results of the conducted research suggest the following key findings: In the early stages of AS, there is an elevation in inflammatory and activity markers, indicating increased inflammation and disease activity. Moreover, IL-17 is identified as a cytokine that may stimulate structural changes, not only in the established and late stages of the disease but also in the early stages.The levels of IL-17 remain elevated in the early stages of AS, suggesting its role in promoting structural changes in the spine and sacroiliac joints. The measurement of MMP-3 can be a valuable laboratory marker for assessing structural progression in AS, particularly in relation to the frequency of erosions.AS involves a dynamic process of new bone formation and inflammation, and an increase in TGFB-1 levels indicates the activation of osteoblasts in AS patients. These findings provide important insights into the pathogenesis and progression of AS and may have implications for the development of targeted therapeutic interventions.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML