R. K. Dadabaeva
Tashkent Medical Academy, Tashkent, Uzbekistan
Correspondence to: R. K. Dadabaeva, Tashkent Medical Academy, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2023 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
The article presents the results of the study of gene polymorphisms involved in the development of metabolically healthy and metabolically complicated obesity in Uzbek women of reproductive age. As a result of the research, the degree of occurrence of alleles and genotypes of the G2548A polymorphism of the LEP gene was evaluated. According to the analysis of the obtained results, in both phenotypes of obesity, carriers of the G allele of the LEP gene G2548A polymorphism and the GG genotype have a higher TVI, which increases their TVI and aggressively affects the development of MAS, while the A allele and AA and GA genotypes are at the I and II levels of obesity. relatively more common means that these alleles and genotypes have a protective effect against increased TVI. Early detection of defects in this polymorphism allows the identification of risk groups for the development of metabolic complications in obese patients and makes it possible to carry out personalized preventive measures in them.
Keywords:
Obesity phenotypes, Metabolically healthy obesity, Metabolically complicated obesity, LEP gene, G2548A polymorphism
Cite this paper: R. K. Dadabaeva, Issues of Genetic Determination of Metabolically Complicated Obesity in Child-Bearing Age Women, American Journal of Medicine and Medical Sciences, Vol. 13 No. 10, 2023, pp. 1398-1402. doi: 10.5923/j.ajmms.20231310.08.
1. Introduction
Many studies have proven the impossibility of a one-size-fits-all approach to determining optimal diets for overweight and obese patients. What has been shown in various works devoted to the study of gene polymorphisms associated with obesity and their interaction [5].Apart from genetic factors, human leptin is encoded by the leptin gene on chromosome 7q32.1 [2,3]. It is highly polymorphic, and a number of single-nucleotide polymorphisms (SNPs) have been found in promoters, exons, and introns regions of this gene [1,4]. Several studies have reported the associations of human leptin gene with obesity, insulin resistance, and diabetes mellitus across different population [3,5,7]. From which, leptin promoter G2548A (rs7799039: guanine > adenine) polymorphism was reported to be associated with increased leptin level, obesity, breast cancer, insulin resistance, and T2DM in many populations [9]. The most common causes of obesity are eating disorders, genetic predisposition, physical inactivity, endocrine system disorders, and environmental factors. There is evidence of a clear relationship between high consumption of sugary drinks and weight gain. Since 1990, there has been a significant increase in the number of people with obesity, primarily associated with the promotion of sugary carbonated drinks. A combination of moderate physical activity and a dietary change to less than 30% total fat, including less than 10% saturated fat, resulted in long-term excess weight loss, according to a study in Finland on diabetes prevention [2,3,8]. The PPARG gene consists of 9 exons and 8 introns and is located on chromosome 3p25. There are 2 isoforms of the PPARG protein: PPARG1 and PPARG2, which differ from each other by the presence of a 28-amino acid site at the N-terminus of PPARG2. PPARG1 is expressed in almost all tissues of the body, and PPARG2 is expressed to a greater extent in adipose tissue. PPARG activation is known to increase adipogenesis and adipocyte differentiation. In macrophages, PPARG is involved in suppressing the production of pro-inflammatory cytokines and increasing tissue susceptibility to insulin, and in the liver and skeletal muscles, in the metabolism of glucose and lipids.A more common mutation of the PPARG gene is considered to be a single-nucleotide substitution of cytosine for guanine in codon 12 (exon B) (rs1801282), as a result of which proline is replaced by alanine (Pro12Ala) in the PPARG2 protein, which leads to a decrease in the transcriptional activity of target genes, including leptin. , a peptide hormone that regulates energy metabolism, resistin, an adipose tissue hormone that controls insulin sensitivity of cells, and an inhibitor of plasminogen activation 1 [7].
2. Aim of the Study
The aim of this work was to establish the role of the G2548A polymorphisms of the LEP gene, the GLY482SER polymorphism of the PPARGC1A gene and the ARG223GLN polymorphism of the LEPR gene in the development of metabolically complicated obesity.
3. Material and Methods
The study was conducted among people of the Caucasian race, the indigenous people of Uzbekistan. 2 groups of patients were examined: the main group of 133 obese patients with complicated metabolic syndrome (OMS), 45 practically healthy individuals as a control group, comparable with the main groups by sex and age. The mean age of the main group was 42.0±0.5 years and the control group 43.2±0.8 years. Criteria for exclusion from the study:• relatives of persons included in the survey;• pregnancy.Molecular genetic testing of DNA was performed by polymerase chain reaction in blood and included the study of the Gly482Ser polymorphism of the PPARGC1A gene.Statistical processing of the obtained data was carried out using the program Statistica 6.1. Genotypes and alleles were analyzed using the chi-square test (%2). Differences were considered statistically significant at p < 0.05. The odds ratio (OR) and relative risk (RR) of developing OOMS in the presence of different alleles and genotypes were calculated. To determine the frequencies of allelic variants of genes, the Hardy–Weinberg law and the Hardy–Weinberg formula were used.
4. Results
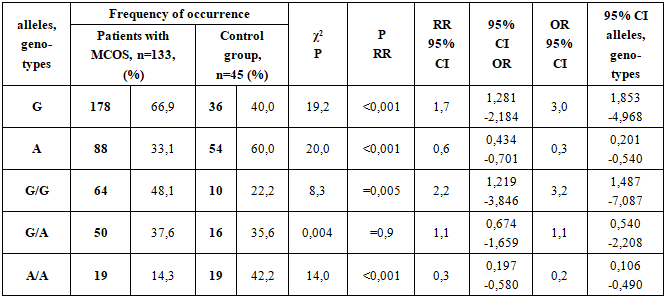
When analyzing the body mass index in the studied groups, it was found that in the main group it was 37.4±0.3 kg/m2, and 25.4±0.3 kg/m2 in the control group, respectively (P<0.001).The prevalence of genotypes of the G2548A polymorphism of the LEP gene in the studied groups is presented in Table 1.Table 1. The prevalence of alleles and genotypes of the G2548A polymorphism of the LEP gene in patients with metabolically complicated obesity syndrome (MCOS) and in the control group
 |
| |
|
The risk of encountering the G allele in patients with MCOS was 2.023 and in controls was 0.667, that is, this allele was reliably 3.0 times more frequent in patients than in controls. This result indicates that the G allele has a reliable aggressive characteristic for the development of MCOS (χ2=19,2; RR=1,7; CI 95%-1,281-2,184; OR=3,0; CI 95%-1,853-4,968; p<0,001).The risk of encountering the A allele in patients with MCOS was 0.494 and in controls was 1.500, that is, this allele was 3.0 times more likely to occur in patients with MCOS than in controls. This result means that the frequency of allele A in healthy people has a reliable protective property against the development of MCOS (χ2 = 20,0; RR=0,6; CI 95% - 0,434-0,701; OR=0,3; CI 95% - 0,201-0,540; p<0,001).G/G genotype as a risk factor for the development of this disease in patients diagnosed with MCOS was 0.928, and in the control group, this indicator was 0.286, that is, in patients diagnosed with MCOS, this genotype occurred 3.2 times more reliably than in healthy individuals. This result indicates that the G/G genotype has a reliable aggressive characteristic for the development of MAS (χ2=8,3; RR=2,2; CI 95%-1,219-3,846; OR=3,2; CI 95%-1,487-7,087; p<0,05).G/A genotype as a risk factor for the development of this disease in patients diagnosed with MCOS was 0.602, in the control group this indicator was 0.552, that is, in patients diagnosed with MCOS, this genotype was 1.1 times more common than in healthy individuals, but this difference was not reliable (χ2=14,0; RR=0,3; CI 95%-0,197-0,580; OR=0,2; CI 95%-0,106-0,490; p<0,001).A/A genotype as a risk factor for the development of this disease in patients diagnosed with MCOS was 0.167, and in the control group, this indicator was 0.731, that is, in healthy individuals, this genotype occurred 4.4 times more reliably than in patients diagnosed with MCOS. This result shows that the A/A genotype has a reliable protective property against the development of MCOS (χ2=14,0; RR=0,3; CI 95%-0,197-0,580; OR=0,2; CI 95%-0,106-0,490; p<0,001).The prevalence of genotypes of the Gly482Ser polymorphism of the PPARGC 1A gene in the studied groups is presented in Table 2.Table 2. The prevalence of alleles and genotypes of the Gly482Ser polymorphism of the PPARGC1A gene in patients with obese complicated metabolic syndrome (OMS) and in the control group
 |
| |
|
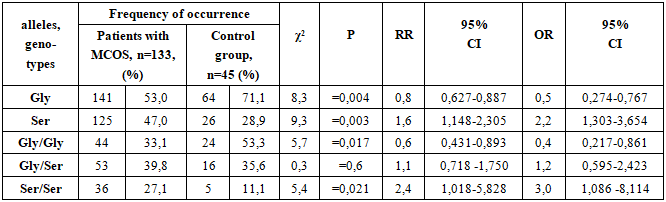
The risk of occurrence of the Gly allele in patients with MCOS was 1.128, and in the control group it was 2.462, that is, this allele was significantly more common in healthy individuals 2.2 times more often than in patients. This result indicates that the Gly allele has a reliable protective property against the development of MCOS (χ2 = 8,3; RR=0,8; 95% CI - 0,627-0,887; OR=0,5; 95% CI - 0,274-0,767; p=0,004).The risk of occurrence of the Ser allele in patients with MCOS was 0.887, and in the control group it was 0.406, that is, this allele was found in patients 2.18 times more often than in controls. This result indicates that the Ser allele has a significant aggressive property for the development of MCOS (χ2 = 9,3; RR = 1,6; 95% CI - 1,148-2,305; OR = 2,2; 95% CI - 1,303-3,654; p = 0,003).The occurrence of the Gly/Gly genotype as a risk factor for the development of this disease in patients with MCOS was 0.494, and in the control group this figure was 1.143, that is, in healthy individuals, this genotype occurred 2.3 times more reliably than in patients with OOMS. This result shows that the Gly/Gly genotype has a reliable protective property against the development of MCOS (χ2 = 5.7; RR=0.6; 95% CI 0.431-0.893; OR=0.4; 95% CI - 0.217-0.861; p = 0.017).The occurrence of the Gly/Ser genotype as a risk factor for the development of this disease in patients diagnosed with MCOS was 0.662, and in the control group this figure was 0.552, i.e., although this genotype occurs 1.2 times more often in patients diagnosed with MCOS than in healthy individuals, this difference is not significant (χ2 = 0.3; RR=1.1; 95% CI - 0.718 -1.750; OR=1.2; 95% CI - 0.595-2.423; p=0.6).The Ser/Ser genotype as a risk factor for the development of this disease in patients diagnosed with MCOS was 0.371, and in the control group this figure was 0.125, that is, in patients with a diagnosis of MCOS, this genotype occurred 3.0 times more often than in healthy individuals. This result indicates that the Ser/Ser genotype has a significant aggressive property for the development of MCOS (χ2 = 5.4; RR=2.4; 95% CI -1.018-5.828; OR=3.0; 95% CI 1.086- 8.114; p = 0.021).The prevalence of Arg223Gln polymorphism genotypes of the LEPR gene in the studied groups is presented in Table 3.Table 3. The prevalence of alleles and genotypes of Arg223Gln polymorphism of the LEPR gene in patients with obese complicated metabolic syndrome (OMS) and in the control group
 |
| |
|
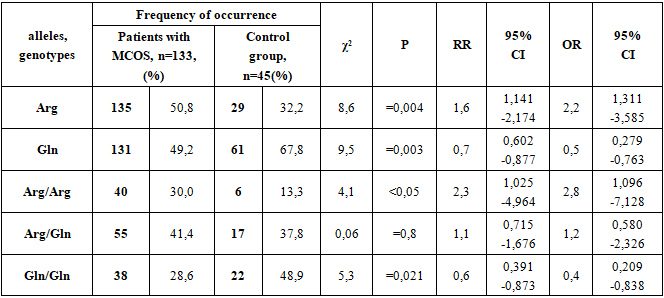
The risk of encountering the Arg allele in patients with MCOS was 1.031 compared to 0.475 in the control group, i.e., this allele was found in patients 2.2 times more often than in the control group. This result indicates that the Arg allele has a reliable aggressive property for the development of MCOS (χ2=8.6; RR=1.6; 95%-1.141-2.174 CI; OR=2.2; 95%-1.311-3.585 CI; p=0.004).The risk of the Gln allele was 0.970 in patients with MCOS and 2.103 in controls, i.e., this allele was 2.17 times more common in patients with MCOS than in controls. This result means that the frequency of the Gln allele in healthy people has a reliable protective effect against the development of MCOS (χ2 = 9.5; RR=0.7; 95% CI - 0.602-0.877; OR=0.5; 95% CI - 0.279 -0.763). (p=0.003).The Arg/Arg genotype as a risk factor for the development of this disease in patients diagnosed with MCOS was 0.430, and in the control group this figure was 0.154, that is, in patients with a diagnosis of MCOS, this genotype occurred 2.8 times more reliably than in healthy individuals. This result indicates that the Arg/Arg genotype has a significant aggressive characteristic for the development of MCOS (χ2=4.1; RR=2.3; 95% CI - 1.025-4.964; OR=2.8; 95% CI - 1.096 -7.128, p < 0.05).The occurrence of the Arg/Gln genotype as a risk factor for the development of this disease in patients diagnosed with OOMS was 0.705, and in the control group this figure was 0.607, that is, in patients with a diagnosis of OOMS, this genotype occurred 1.16 times more often than in healthy individuals, but this difference was not significant (χ2=0.06; RR=1.1; 95% CI - 0.715-1.676; OR=1.2; 95% CI - 0.580-2.326; p=0.8).The Gln/Gln genotype as a risk factor for the development of this disease in patients diagnosed with MCOS was 0.400, and in the control group this figure was 0.957, that is, in healthy individuals, this genotype occurred 2.4 times more often than in patients diagnosed with MCOS. This result indicates that the Gln/Gln genotype has a strong protective property against the development of MCOS (χ2=5.3; RR=0.6; 95%-0.391-0.873 CI; OR=0.4; 95%-0.209-0.838 CI ; p=0.021).
5. Conclusions
Summarizing, according to the obtained results, the Gly482Ser polymorphism of the PPARGC1A gene was associated with the development of metabolically complicated obesity, based on the more frequent occurrence of the Ser mutation allele (2.18 times more often) than the group of healthy individuals, the heterozygous Gly/Ser genotype (1.2 times more often) and mutational genotype Ser/Ser (3.0 times more often).The G2548A polymorphism of the LEP gene was associated with MCOS, the mutation allele A was significantly more common in the group of patients with obesity compared to the control group (60% vs. 33.1%), this pattern was not characteristic of the mutational genotype.With regard to the Arg223Gln polymorphism of the LEPR gene, an association of MCOS with the normal Arg allele (2.2 times more often), with the normal homozygous Arg/Arg genotype (2.8 times more often) was determined. Based on the obtained results, it can be concluded that there is a risk of developing MCOS in patients with defects in the Gly482Ser polymorphism of the PPARGC1A gene, whose mutational alleles and genotypes are highly associated with this category of patients. Early determination of the presence of defects in this polymorphism will create an opportunity to identify risk groups for the development of metabolic complications in patients with obesity and will allow for personalized preventive measures.
References
| [1] | Borodina C.B., Gapparova K.M., Zaynudinov Z.M., Grigor'yan O.N. Geneticheskiye prediktory razvitiya ozhireniya // Ozhireniye i metabolizm. - 2016. - T.13. - №2. - S. 7-13. doi:10.14341/OMET201627-13.1. |
| [2] | Dadabaeva R.К., Gadaev A.G., Kurbanov A.K. «Метаболик синдром ва семизликни ривожланишининг замонавий жиҳатлари»// New day in medicine. Bukhara.– 2023. - № 1 (51), Р.246-250. |
| [3] | Dadabaeva R.К., Gadaev A.G., Kurbanov A.K. «Семизликни ривожланиши ва уни бошқарилишида биологик фаол можддаларнинг тутган ўрни»// Journal of Theoretical and Clinical Medicine. Tashkent/ – 2023. - № 1, Р.35-42. |
| [4] | Enns JE, Taylor CG, Zahradka P. Variations in adipokine genes AdipoQ, Lep, and LepR are associated with risk for obesity-related metabolic disease: the modulatory role of gene-nutrient interactions. J Obes. 20112011. |
| [5] | http://www.who.int/mediacentre/factsheets/fs311/ru/. Ссылка активна на 29.06.2016. [Obesity and overweight. Fact sheet. Updated June 2016. WHO Media centre. Available on: http://www.who.int/mediacentre/factsheets/fs311/en/]2. |
| [6] | Li K, Liu Y, Venners SA, Hsu YiH, Jiang S, Weinstock J, et al. Effects of LEP G2548A and LEPR Q223R polymorphisms on serum lipids and response to simvastatin treatment in Chinese patients with primary hyperlipidemia. Clin Appl Thromb Hemost. 2017; 23: 336-44. |
| [7] | Mammès, O., Betoulle, D., Aubert, R., Herbeth, B., Siest, G., & Fumeron, F. (2000). Association of the G-2548A polymorphism in the 5′ region of the LEP gene with overweight. Annals of Human Genetics, 64(5), 391-394. doi:10.1017/S0003480000008277.3. |
| [8] | Shabana Hasnain S. Leptin promoter variant G2548A is associated with serum leptin and HDL-C levels in a case control observational study in association with obesity in a Pakistani cohort. J Biosci. 2016; 41: 251–5. |
| [9] | Yu Z, Han S, Cao X, Zhu C, Wang X, Guo X. Genetic polymorphisms in adipokine genes and the risk of obesity: a systematic review and meta-analysis. Obesity. 2012; 20: 396–406. |


 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML

