-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2023; 13(9): 1346-1348
doi:10.5923/j.ajmms.20231309.38
Received: Aug. 15, 2023; Accepted: Sep. 10, 2023; Published: Sep. 28, 2023

Metabolic Disorders in Patients with Ovarian Hyperandrogenia with Aplasia of the Uterus and Vaginus
Adilova M. N., Negmadjanov B. B., Rabbimova G. T.
Samarkand Medical University, Uzbekistan
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

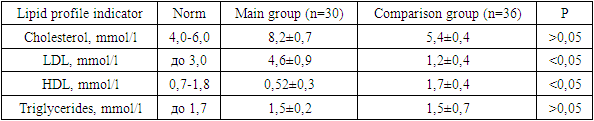
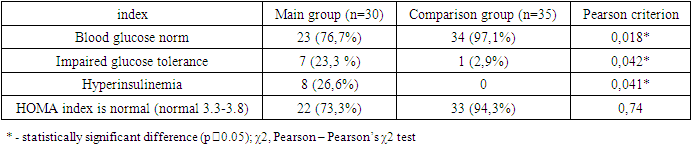
The article provides data on carbohydrate and lipid metabolism in women with aplasia of the vagina and uterus. The main manifestation of disorders of lipid and carbohydrate metabolism was an increase in the level of LDL 3.8 times higher in patients with aplasia of the vagina and uterus in combination with hyperandrogenism, and the level of HDL was 3.3 times lower than normal values, this indicates a violation of lipid metabolism in patients with the main groups; blood glucose levels and impaired glucose tolerance differed statistically significantly between the main group and the comparison group. In addition, insulin levels and the presence of hyperinsulinemia were also statistically significantly different between the two groups.
Keywords: Aplasia of the vagina and uterus, Hyperandrogenism of ovarian origin, Insulin resistance, Lipid metabolism
Cite this paper: Adilova M. N., Negmadjanov B. B., Rabbimova G. T., Metabolic Disorders in Patients with Ovarian Hyperandrogenia with Aplasia of the Uterus and Vaginus, American Journal of Medicine and Medical Sciences, Vol. 13 No. 9, 2023, pp. 1346-1348. doi: 10.5923/j.ajmms.20231309.38.
1. Introduction
- Congenital uterovaginal aplasia or aplasia Mayer-Rokitansky-Küster-Hauser syndrome (MRKH) is characterized by unfused uterine buds, aplasia of the cervix and vagina, but normal or hypoplastic bilateral adnexa and is clinically manifested by primary amenorrhea. Patients with MRKH have a normal developmental female phenotype and karyotype (46,XX) and an incidence of 1 in 4000 or 5000 births (Cheroki et al. 2006) [11-14]. Aplasia of the vagina and uterus is a rare condition in which the vagina and uterus do not develop properly. This can lead to a range of physical and psychological problems, including difficulty having sex, menstrual irregularities and infertility. Aplasia of the vagina and uterus can occur as an isolated condition or in combination with other diseases, such as ovarian hyperandrogenism. Ovarian hyperandrogenism is a condition in which the ovaries produce excessive amounts of androgens such as testosterone. This can lead to a range of symptoms including acne, hirsutism and menstrual irregularities. Ovarian hyperandrogenism is often associated with polycystic ovary syndrome (PCOS), which is a common hormonal disorder affecting women of reproductive age [1,11-14].Despite the significant impact of vaginal and uterine aplasia in combination with ovarian hyperandrogenism on women's health, there is currently no consensus on the optimal approaches to the diagnosis and treatment of this condition. This highlights the need for further research and study on this topic. In addition, identifying new approaches to diagnosing and treating this condition may also have wider social and economic implications. By improving women's health and reproductive outcomes, we can help reduce the burden of infertility, reduce health care costs associated with suboptimal management, and improve the overall well-being of patients and their families. Hyperandrogenism is associated with an increased risk of cardiovascular disease, including atherosclerosis and high blood pressure, as well as insulin resistance, which can lead to type 2 diabetes in the future [2-10].The purpose of the study was to study the characteristics of carbohydrate and fat metabolism in patients with aplasia of the vagina and uterus in combination with hyperandrogenism of ovarian origin.
2. Material and Research Methods
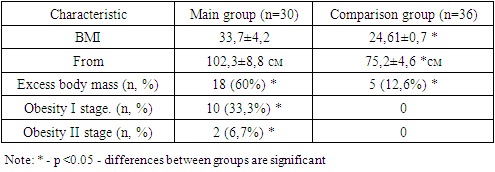
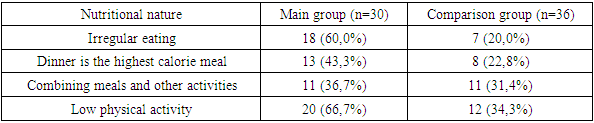
- The studies were carried out in the department of pediatric gynecology of a multidisciplinary medical center and the private company “Doctor Shifo Bakht”. Clinical data and sonographic results of 65 patients with Rokitansky-Küster-Hauser-Mayer syndrome were studied. The patients were divided into 2 groups: 1st - main group of patients with aplasia of the vagina and uterus in combination with signs of hyperandrogenism - 30 patients, 2nd group - comparison 35, women with aplasia of the vagina and uterus without signs of hyperandrogenism.The criteria for inclusion in the study were: 1) the presence of 2 of the 3 listed signs: androgen-dependent dermopathy (acne and hirsutism), hyperandrogenemia (increased levels of androgens in the blood); signs of ovarian hyperandrogenism based on ultrasound results. Exclusion criteria: 1) previous treatment with hormonal drugs that affect steroidogenesis in the ovaries or adrenal glands; 2) the presence of diseases that provoke the development of secondary hyperandrogenism (type 1 diabetes mellitus, hypercortisolism, hypothyroidism); 3) taking medications whose side effect is the development of hyperandrogenism and anovulation (valproic acid, etc.). The patients underwent a comprehensive examination, including general clinical, hormonal, echographic and morphological methods, on the basis of which the form of hyperandrogenism syndrome was determined. Patients in the comparison group had an average BMI of 24.61±0.7 and an average age of 29.6±1.36 years; patients with MRKH in combination with hyperandrogenism had a BMI of 33.7±0.5; the average age was 28.2±1. 23 years old. During an objective examination of the patients of the main group, attention was paid to skin symptoms of endocrine dysfunction (purple, pink and white stretch marks on the skin of the abdomen, shoulders, mammary glands, thighs, negroidity in specific areas). Striae of varying severity, as a sign of previous or current hypercortisolism, were detected in 6 (20%) of the main group and only in 2 (5.7%) of the comparison group. Clinical signs of hyperandrogenism were identified in 18 (60%) women of the main group. Symptoms of hyperandrogenism were manifested by acne vulgaris and oily seborrhea in 12 (40%), hirsutism in 13 (43.3%) patients. Hirsutism was predominantly in girls of the main group and was detected in varying degrees of severity in 13 (43.3%), which was almost 7 times more often than in the comparison group (5.7%). Moreover, severe hirsutism was present in 4 (13.3%) of the main group, while in the comparison group it was not detected in any of them. Patients of the main group 16 (53.3%) complained of headaches three times more often than patients of the comparison group. Data on examination of body weight, BMI and eating behavior in patients with aplasia of the vagina and uterus in combination with hyperandrogenism of ovarian origin. Considering the heterogeneity of the group in terms of the severity of obesity, patients in both groups were divided according to the degree of obesity by BMI and waist circumference (WC) to diagnose metabolic disorders, and subsequently to assess the effectiveness of treatment. (table 1).
|
|
|
|
|
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML