-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2023; 13(9): 1343-1345
doi:10.5923/j.ajmms.20231309.37
Received: Aug. 27, 2023; Accepted: Sep. 19, 2023; Published: Sep. 28, 2023

Bioactive Coating and Sterility: Analyzing the Implant.Uz Dental Implant
F. K. Usmonov, N. L. Khabilov, T. O. Mun
Tashkent State Dental Institute, Uzbekistan
Correspondence to: F. K. Usmonov, Tashkent State Dental Institute, Uzbekistan.
| Email: |  |
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Air is one of the most significant factors of microbial pollution. Usually, mechanical particles that pollute the air are carriers of microflora. The results of a microbiological study of the domestic dental implant Implant.Uz with a bioactive coating after radiation are considered equal. Prior to sterilization, the implant was packaged. Studies have revealed the best dosage of radiation for sterilization of the domestic dental implant Implant.Uz with a bioactive coating.
Keywords: Sterilization, Radiation, Dental implant, Microbiological research
Cite this paper: F. K. Usmonov, N. L. Khabilov, T. O. Mun, Bioactive Coating and Sterility: Analyzing the Implant.Uz Dental Implant, American Journal of Medicine and Medical Sciences, Vol. 13 No. 9, 2023, pp. 1343-1345. doi: 10.5923/j.ajmms.20231309.37.
1. Introduction
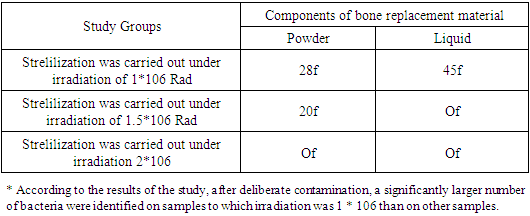
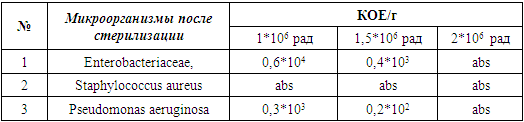
- Infection control procedures have become an integral part of modern dentistry and have had a huge impact on all clinical practice. There is not much current research on infection control procedures aimed at reducing the number of microbes on dental materials consisting of powder and liquid. There is also little research data on the radiation method of sterilization, which more often leads to satisfactory results.The purpose of the study was to find the best dosage for sterilization of the domestic dental implant Implant.Uz with a bioactive coating. During the course of the study, special attention was paid to preventing the sealing of packaged test materials from breaking. After introducing the strains into Eppendorf tubes, all experimental procedures were carried out in an anaerobic chamber, which guaranteed an optimal environment for the growth of the three bacterial species mentioned above. The task was set to study the effectiveness of the procedure for radiation sterilization of dental material, consisting of powder and liquid, extracted from the original packaging, for the presence of bacteria.Radiation studies were carried out at the Institute of Nuclear Physics M.Yu. Tashmetov. In the present study, 65 implants were used. All samples were placed in sealed bags for 12 minutes and subjected to steam treatment for radiation sterilization at 1*106 Rad, 1.5*106 Rad and 2*106 Rad at a temperature in the irradiation zone of 20°C. (Figure 1 and 2)
 | Figure 1. Radiation devices UIM2-2D, DKG-02U |
 | Figure 2. Sterilized materials in sealed packaging |
2. Determination of the Number of Bacteria
- The sterility test is carried out under aseptic conditions, in boxes, preferably under a sterile laminar air flow, wearing sterile antistatic clothing. 2 hours before the start of work, bactericidal lamps were turned on in the box to disinfect the air and surfaces. The air in the box was regularly checked for microbial contamination. To do this, Petri dishes with MPA, Sabouraud's medium and thioglycollate (mercaptoacetic) medium are left open for 15 minutes, then closed and kept in a thermostat at 37°C for 48 hours. There should be no more than 5 colonies on the dish; a larger number indicates high contamination of the box. There should be no mold or yeast in the air. Work in boxing is carried out in sterile gowns and slippers.The sterilized powder was scattered over the entire surface of the dish with medium M009 at a temperature of 32°C and M013 at a temperature of 20-22°C and incubated for seven days, and then their contents were examined for the presence of bacteria.To determine microbial contamination, non-injectable medicinal products are subjected to bacteriological examination to determine the amount of saprophytic bacteria, yeast and mold fungi, as well as the presence of bacteria of the Enterobacteriaceae genera, Staphylococcus aureus species, and Pseudomonas aeruginosa.The crops were inspected daily. If there were no microorganisms in all test tubes, a conclusion was made that the dental material was sterile; if there were signs of microflora growth in the test tubes, the material was considered not sterile.For each sample, the average number of colony-forming units (CFU) per 1 milliliter of solution (CFU/ml) was calculated. Multiple linear regressions were used to compare the effectiveness of different radiation doses. Differences at the p < 0.05 level were considered statistically significant.Results of a study of the sterility of components of the domestic dental implant Implant.Uz with a bioactive coating Table 1 and 2.
|
|
3. Conclusions
- The results of this study indicate that differences in radiation dosage affect the effectiveness of sterilization, with samples of the studied object more often showing complete absence of bacteria. The best results of sterilization efficiency were observed when using 2*106 rad radiation.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML