-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2023; 13(9): 1325-1331
doi:10.5923/j.ajmms.20231309.33
Received: Aug. 13, 2023; Accepted: Sep. 7, 2023; Published: Sep. 28, 2023

Comparative Analysis of the Level of Neurotrophic Factors in Children with Intracranial Hemorrhages
Usmanova P. T.
Tashkent Institute of Postgraduate Medical Education, Tashkent, Uzbekistan
Correspondence to: Usmanova P. T., Tashkent Institute of Postgraduate Medical Education, Tashkent, Uzbekistan.
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Objective: The aim of this study is to study the prognostic significance of neurotrophins as markers for the early detection of intraventricular hemorrhage. Materials and Methods: The study included 156 children with intracranial hemorrhages (intraventricular hemorrhages, subarachnoid hemorrhage and parenchymal hemorrhages. Results: 156 patients were divided into 4 groups: group 1 - 35 (22.4%) newborns diagnosed with IVH stage 1-2 (all indicators of this group were used to compare the data obtained and the adequacy of the results); group 2 - 32 (30.2%) children diagnosed with IVH stage 3-4; group 3 - 10 (6.4%) children with SAH; group 4 - 79 (49.2%) infants with PH. Statistically significantly low levels of CNTF and pNT-H were found in the IVH 1-2 group (32.2±24.4 ng/ml and 257.0±292.0 ng/ml) compared with other groups. The highest values of CNTF were in the IVH 3-4 group (110.2±57.7 ng/ml), and pNT-H1 in the SAH group (1149.8±2692.0 ng/ml). There is a decrease in the levels of CNTF (66.2±54.6 ng/ml) and pNT-H1 (644.1±1364.7 ng/ml) after 3-6 months. up to 58.3±46.7 ng/ml and 230.3±238.9 ng/ml respectively. After 2 years, there was a further decrease in the concentration of neurotrophins to 34.5±34.8 ng/ml (CNTF) and to 134.7±128.0 ng/ml (pNT-H1). It was determined that the optimal cut-off point for CNTF is 51.3 ng/ml, for pNT-H - 186.7 ng/ml. Conclusion: Low values of CNTF and pNT -H are significantly more often recorded in the group with a favorable outcome than in the group with a moderate and unfavorable outcome. In the recovery period, almost half (42.9%) of patients without neurological deficit had low concentrations of CNTF and pNT -H, while 32.1% of children with an initially elevated level of neurotrophins moved into the category of severe deficiency. During the recovery period, 6.2% of patients with initially severe neurological deficits but low levels of CNTF and pNT -H had no impairments, while 74.2% of patients with high concentrations of neurotrophins had severe neurological impairments.
Keywords: Intraventricular hemorrhages, Subarachnoid hemorrhage, Parenchymal hemorrhages, Neurotrophic factors
Cite this paper: Usmanova P. T., Comparative Analysis of the Level of Neurotrophic Factors in Children with Intracranial Hemorrhages, American Journal of Medicine and Medical Sciences, Vol. 13 No. 9, 2023, pp. 1325-1331. doi: 10.5923/j.ajmms.20231309.33.
1. Introduction
- Violation of cerebral circulation (ICC) in the form of intracranial hemorrhages (ICH) is an extremely serious pathology not only for adults, but also for children, associated with the life of a child at any age [Ballabh P. 2017].Intracranial hemorrhages are observed in 30% of children under the age of 2 months, in 50% under 1 year, and only in 1% in adolescents aged 15-18 years [Romanova E.O., 2019]. According to Mackay M. et al. [2016] 10% of newborns die in the first week from a hemorrhagic stroke, 40% of children have no consequences, and the remaining 60% have serious neurological disorders throughout their lives.Unfortunately, even the use of highly informative methods for diagnosing perinatal intracranial injuries, such as neurosonography (NSG), computed tomography, diffusion-tensor magnetic resonance imaging (tractography), dopplerography, electroencephalography, cannot always detect and timely diagnose the onset of pathological changes occurring on different levels and stages of CNS disease [Morgun A.V., 2013].Therefore, the search for early markers of CNS damage and the simplification of their interpretation is certainly relevant. Biomarkers can be roughly divided into markers of neuronal damage, markers of neuroglia damage, and other markers depending on the characteristics of damaged cells [Yao M., 2019].It is known from the literature that ciliary neurotrophic factor (Ciliary neurotrophic factor - CNTF) increases rapidly and significantly after traumatic or ischemic injury and plays an important role in neurogenesis and differentiation of neural stem cells [Ding J., 2013; Kang S., 2012], and an increased level of neurotrophin can induce apoptosis [Harada T., 2002].The main white matter components integrated into the cytoskeleton of axons are neurofilaments, which can serve as a biomarker nerve tissue damage [Sellner J., 2011; Tao C., 2017]. To the features of neurofilament pNF-H include: release after acute intracerebral hemorrhage; high predictive value for long-term outcome and early neurological deterioration, and being an independent predictor of long-term outcome and early neurological deterioration [Cai J., 2013].The purpose of the study was to study the prognostic significance of neurotrophins as markers for the early detection of intraventricular hemorrhage.
2. Materials and Methods
- The study included 156 children with intracranial hemorrhages (intraventricular hemorrhages (IVH), subarachnoid hemorrhage (SAH) and parenchymal hemorrhages (PH)), who were hospitalized in the intensive care unit and the microneurology department of the City Clinical Children's Hospital No. 1 in the period from 2017 to 2020, followed by their observation on an outpatient basis at the place residence, with the assistance of the Department of Pediatric Neurology TashIUV.Criteria for inclusion in the study group:- children aged from birth to 2 years with a confirmed diagnosis of "hemorrhagic stroke" and "IVH" according to the international classification;- written consent of parents/guardians to participate in the research;- children in acute, recovery and residual periods of hemorrhagic cerebral stroke;- anamnestic, clinical, neuroimaging and neurophysiological signs confirming vascular lesions of the brain;Criteria for exclusion from the study group:- age of patients older than 2 years;- the presence of severe hereditary pathology, developmental anomalies, degenerative diseases;- the presence of severe concomitant pathology affecting the course of the disease and the results of diagnostic studies.- children with paroxysmal syndromes that do not have neurophysiological confirmation.All young children with intracranial hemorrhages (ICH) underwent in-depth clinical, neurological and paraclinical studies to differentiate diseases similar in clinical course and to choose a strategy for a therapeutic approach. For a detailed study of clinical and neurological signs, when filling out the developed individual card, we included an examination of the neurological status using the PedNIHSS stroke scale and PSOM SNE.Of the instrumental studies, neurosonography (NSG), computed tomography and magnetic resonance tractography of the brain were used.Statistical processing of the results was carried out using Microsoft Excel, MedCalc version 18.5 and easyROC, ver. 1.3.1. The initial data were evaluated for compliance with the normal distribution according to the Kolmogorov-Smirnov criterion. To assess the prognostic significance of markers, ROC analysis was used (Receiver Operating _ Characteristic), with the calculation of the area under the curve AUC (area under the curve), Se (sensitivity) and Sp (specificity) of the model. Results are presented as median (Me) [interquartile range Q25; Q75]. Differences were considered statistically significant at p< 0.05.
3. Results and Discussion
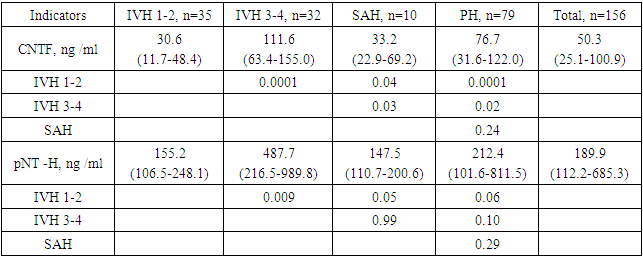
- All patients were divided into 4 groups: group 1 included 35 (22.4%) newborns with a diagnosis of IVH 1–2 transferred and confirmed for NSG (all indicators of this group were used to compare the data obtained and the adequacy of the results); group 2 included 32 (30.2%) children with a diagnosis of IVH 3-4 st; group 3 consisted of 10 (6.4%) children with SAH; Group 4 consisted of 79 (49.2%) young children with PH.The data obtained indicate that in the IVH 1-2 group (32.2±24.4 ng /ml and 257.0±292.0 ng /ml) there are significantly low values of CNTF and pNT-H, respectively, compared with other groups. The highest levels of CNTF were in the IVH 3-4 group (110.2 ± 57.7 ng / ml), and pNT-H1 in the SAH group (1149.8 ± 2692.0 ng / ml) (Table 1.).
|
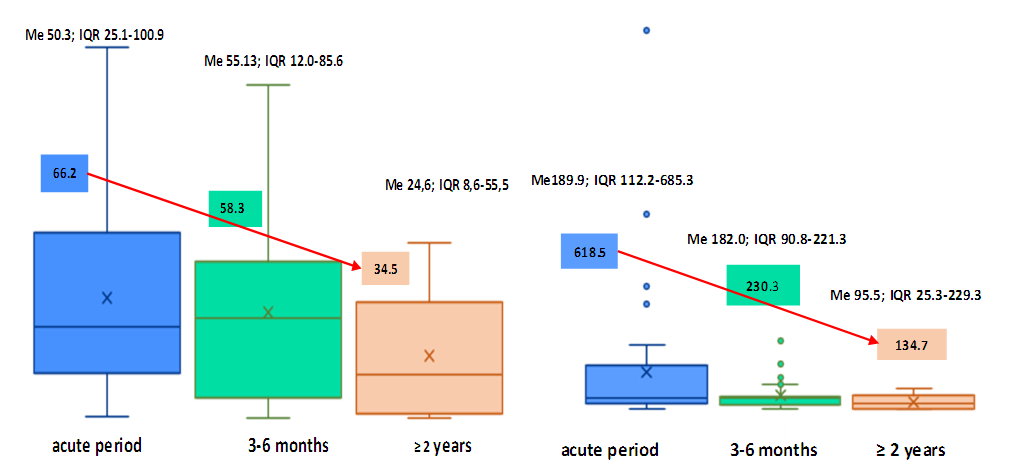 | Figure 1. Dynamics of concentrations of CNTF and p NT -H1 according to the timing of the survey (the arrow indicates the direction of the dynamics), ng / ml |
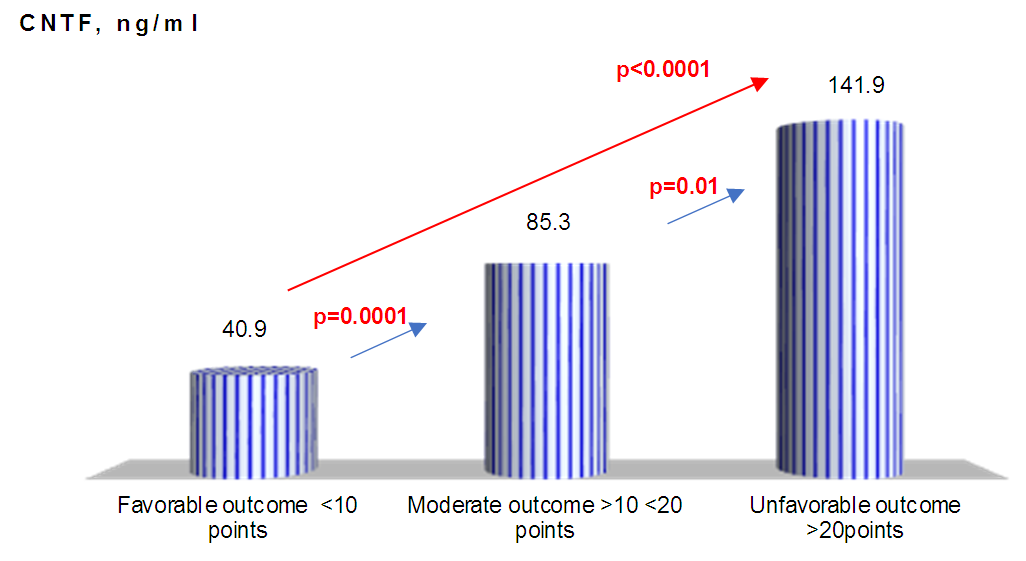 | Figure 2. CNTF concentration relative to stroke severity |
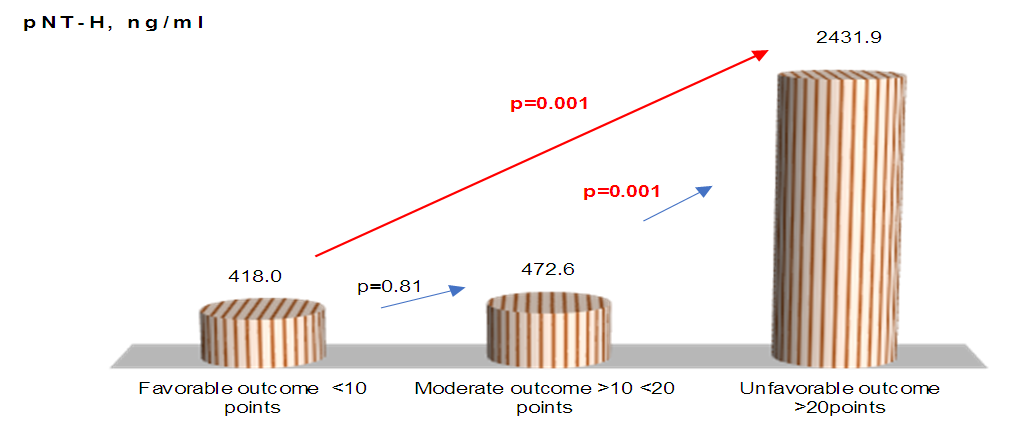 | Figure 3. pNF -H concentrations relative to stroke severity |
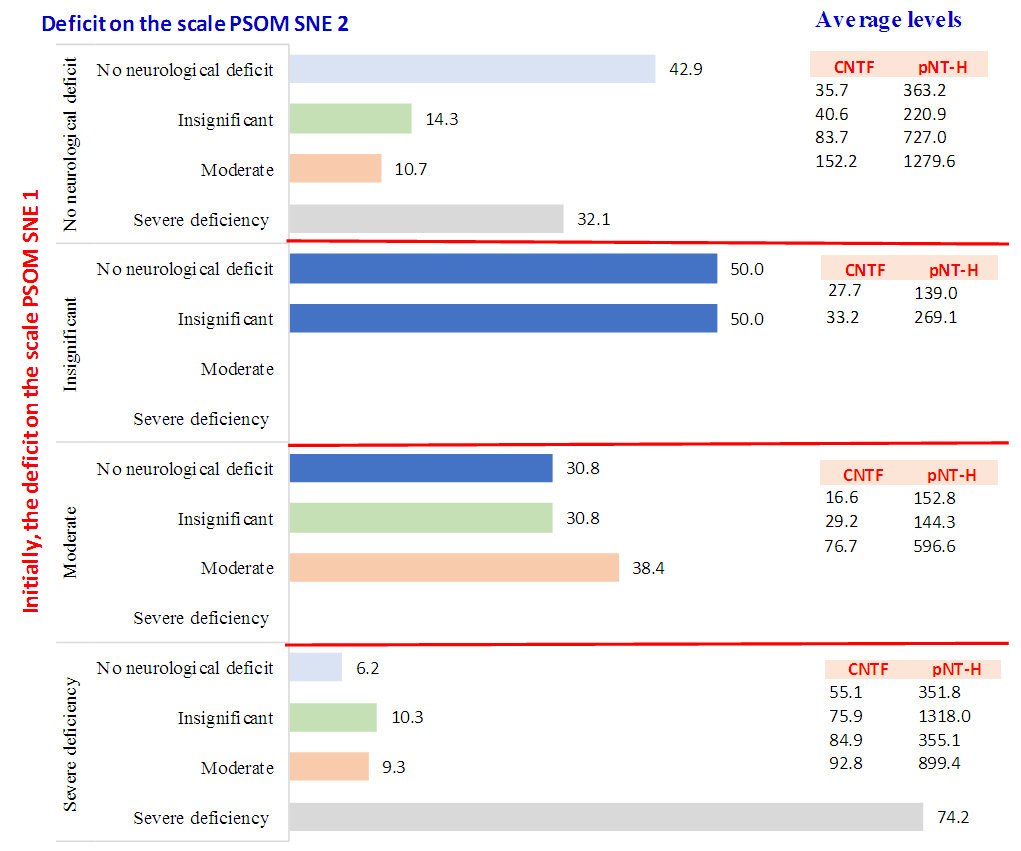 | Figure 4. Level of neurotrophins (CNTF and pNT -H) depending on the degree of neurological deficit |
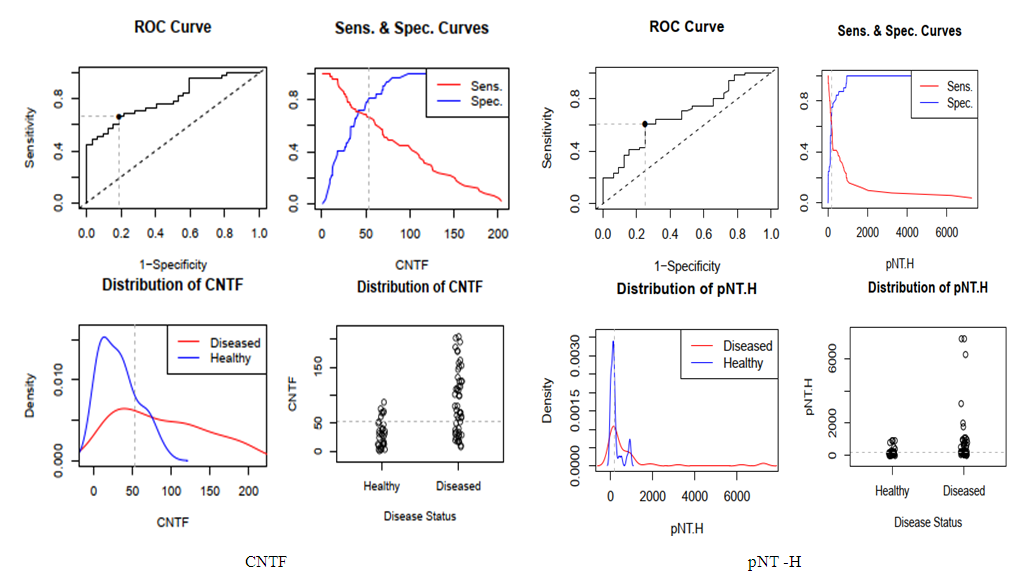 | Figure 5. cut - off point for neurotrophin levels |
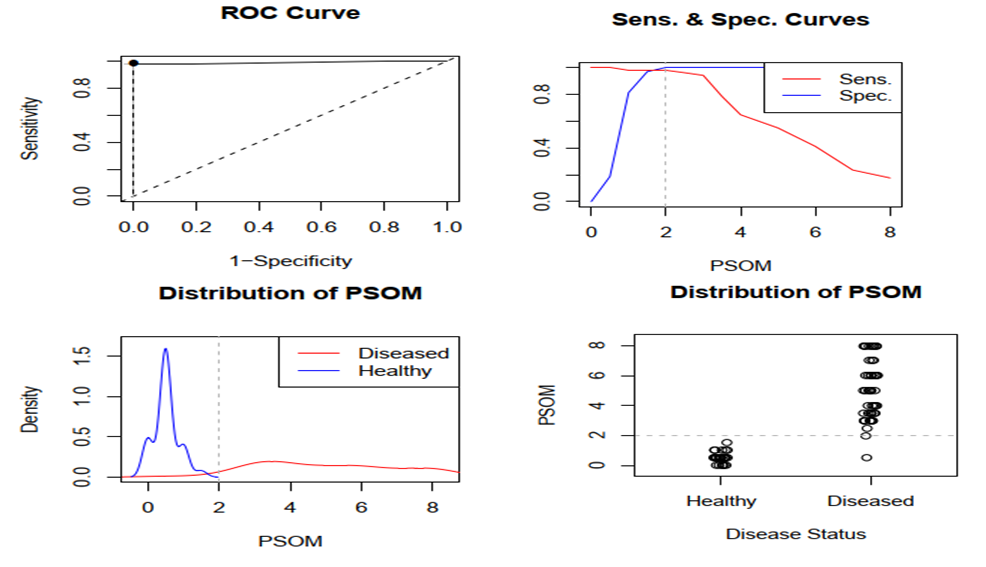 | Figure 6. cut - off point for PSOM-SNE summary |
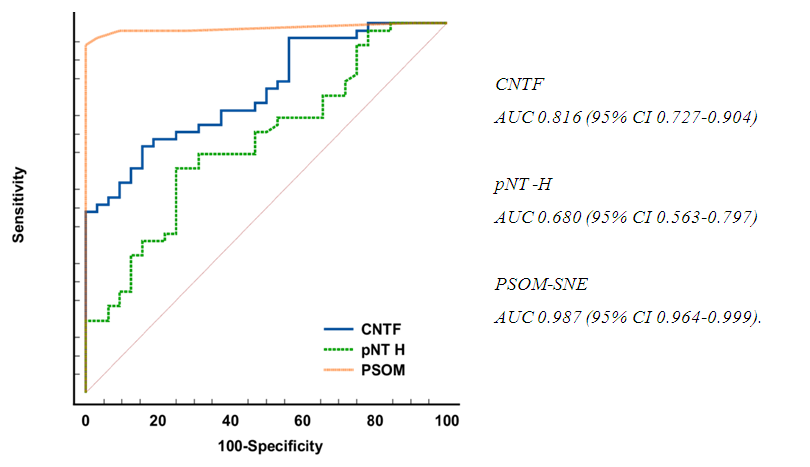 | Figure 7. ROC curve of the prediction model |
4. Conclusions
- Thus, we have established that:the highest levels of CNTF and p NT -H1 are detected in the acute period of stroke, with a significant decrease during the recovery period (after 2 years);low values of CNTF and pNT -H are significantly more often recorded in the group with a favorable outcome than in the group with a moderate and unfavorable outcome ;Initially, the content of CNTF and pNT -H in children without neurological deficit was significantly lower than in patients with moderate and severe deficiency;in the recovery period, almost half (42.9%) of patients without neurological deficit had low concentrations of CNTF and pNT -H, while 32.1% of children with an initially elevated level of neurotrophins moved into the category of "severe deficiency";during the recovery period, 6.2% of patients with initially severe neurological deficits but low levels of CNTF and pNT -H had no impairments, while 74.2% of patients with high concentrations of neurotrophins had severe neurological impairments;the optimal cut-off point for CNTF and pNT -H is 51.3 ng /ml and 186.7 ng /ml, respectively, cut - off point for PSOM-SNE indicator <1 point;neurotrophins CNTF and pNT -H were respectively very good (AUC 0.816) and average (AUC 0.680) predictive value.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML