-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2023; 13(9): 1255-1259
doi:10.5923/j.ajmms.20231309.17
Received: Sep. 10, 2023; Accepted: Sep. 22, 2023; Published: Sep. 23, 2023

Endothelial Dysfunction in Type 2 Diabetes Patients after COVID-19: How Long Should We be Afraid of Thrombosis?
Dilovar Khalilova, Anna Alieva
Republican Specialized Scientific and Practical Centre of Endocrinology
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

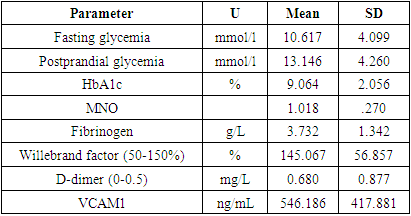
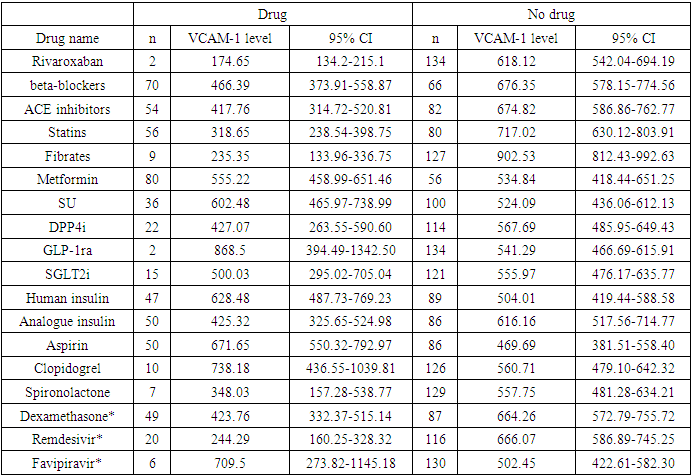
Background. High cardiovascular risk in patients with type 2 diabetes mellitus is enhanced due to endothelial dysfunction during COVID-19. How long does post-Covid damage to endothelial cells persist is still unclear. The aim of our study was to evaluate the level of vascular cell adhesion molecules VCAM-1 as one of the parameters of endothelial function in patients with type 2 diabetes mellitus after COVID-19 infection. Methods. 136 type 2 diabetes patients who had COVID-19 in 2020 were examined in 1-24 months after the acute infection. VCAM-1 was tested using the Human ELISA Kit assay (Elabscience). Statistical analysis was performed using STATA v.16.0. Results. The signs of endothelial dysfunction were present up to 24th months after COVID-19. Patients who received oral anticoagulants had lower level of VCAM-1 174.65 (95% CI 134.2-215.1) comparing to those who did not 618.12 (95% CI 542.04-694.19) or was prescribed antiaggregant therapy (671.65, 95% CI 550.32-792.97 for aspirin and 738.18, 95% CI 436.55-1039.81 for clopidogrel). Use of dexamethasone in acute COVID-19 was associated with lower VCAM-1 levels in post-COVID period (423.76, 95% CI 332.37-515.14 vs 664.26, 95% CI 572.79-755.72). Conclusion. People with type 2 diabetes are at high risk of thrombotic complications due to endothelial dysfunction up to 2 years after COVID-19. Use of oral anticoagulants should be considered in type 2 diabetes patients after COVID-19 under the control of hemostasis for at least one year after COVID-19. The potential benefit of glucocorticoids use in terms of endothelial function should be studied more deeply.
Keywords: Diabetes, COVID-19, Vascular adhesion molecules, Endothelial dysfunction, Dexamethasone, Oral anticoagulant therapy
Cite this paper: Dilovar Khalilova, Anna Alieva, Endothelial Dysfunction in Type 2 Diabetes Patients after COVID-19: How Long Should We be Afraid of Thrombosis?, American Journal of Medicine and Medical Sciences, Vol. 13 No. 9, 2023, pp. 1255-1259. doi: 10.5923/j.ajmms.20231309.17.
Article Outline
1. Background & Objectives
- Diabetes mellitus (DM) is associated with increased cardiovascular events risk. One of the reasons is endothelial dysfunction well described in type 2 DM patients [1]. COVID-19 pandemia although announced to be over, has increased the number of thrombotic complications, especially in patients with type 2 DM due to inflammation of endothelial cells in all organs and vessels [2].Molecules of adhesion are a group of glycoproteins expressed on the cell surface and play a pivotal role in inflammatory and oncological processes and are one of indicators of endothelial dysfunction [3]. Some studies showed the increase of these molecules within several hours after SARS-CoV-2 contamination. The level of molecules of adhesion positively correlates with disease severity and mortality rate [2]. But what if endothelial dysfunction continues even after discharge of virus? And what is the best way to prevent thrombotic complications in patients with type 2 DM on the long-term basis?The objectives of our study was to evaluate the level of vascular cell adhesion molecules VCAM-1 as one of the parameters of endothelial function in patients with type 2 diabetes mellitus after COVID-19 infection.
2. Materials and Methods
- 136 type 2 DM patients were studied in our cross-sectional study. Inclusion criteria were known type 2 DM and COVID-19 in 2020, which was confirmed by PCR during the onset of COVID-19. COVID-19 was classified as mild, moderate, or severe based on the local recommendations on the management of patients with COVID-19. Sampling size was calculated in order to receive data with 95% CI and margin of error of 5%.VCAM-1 was measured in ng/mL using a Human ELISA Kit assay (Elabscience). In brief, blood samples were collected in the fasting state and centrifuged immediately at 1000 rpm for 20 minutes at 2-8°C. Samples and standards were added in a volume of 100 µL onto the bottom of the plate, avoiding touching the wall and foaming, and incubated at 37°C for 90 min. After decanting the liquid, 100 µL of Biotinylated Detection Ab working solution was added and incubated, covered with a sealer, at 37°C for 1 hour. After decanting the solution, the wells were washed thrice with 350 µL of wash buffer. Then 100 µL of HRP Conjugate working solution was added and incubated at 37°C for 30 min, followed by washing five times with buffer. After that, 90 µL of Substrate reagent was added and incubated at 37°C for 15 min, protected from the light. Then, 50 µL of Stop solution was added, and the optical density was measured at once with a micro-plate reader set to 450 nm. The ELISA Kit was used, according to instruction, for research only, not for diagnostic procedures. Ethical approval was taken at the ethical committee of the Republican Centre of Endocrinology #1/A-CC-2021-139.Statistical analysis was performed using STATA v.16.0. Parametric parameters were analyzed using a t-test in case of the normal distribution; the data are shown in mean and standard deviation and were considered statistically significant in p<0.05. Non-parametric criteria were analyzed using χ2 or Fisher exact test and are shown in numbers and/or percent.
3. Results
- In total, we studied 136 type 2 diabetes patients. The time after the acute infection was from 1 to 24 months. The mean age was 59.81±7.96 years (from 43 to 80 years), and 41.18% were male. The mean diabetes duration was 10.38±6.74 years (min 0, max 35 years). The mean BMI was 29.61±5.27 kg/m2.8.08 % of patients had severe COVID-19, 46.46 % - moderate, and 60.61 % - mild COVID-19. 22 (16.18%) patients did not know about the viral disease, had not been vaccinated by the time of the study, and had a high level of neutralizing IgG antibodies to COVID-19. 8.1% of patients were vaccinated for COVID-19 at the time of the study.40.4% patients were overweight, 38.2% were obese. 55.9% had arterial hypertension. Of them 8,1% did not take any antihypertensive therapy. 71.4% of patients received insulin therapy (36.8% - insulin analogues, and 34.6% - human insulins). 58.8% took biguanides, 16.2% DPP4i, 1.5% - GPP1ra, 26.5% SU, 11.0% - SGLT2i.As a treatment of concomitant diseases, patients received: beta-blockers – 51.5%, ACEi – 39.7%, BRA – 7.4%, Ca channel blockers – 16.9%, aspirin – 36.8%, rivaroxaban – 1.5%, clopidogrel – 7.4%, verospirone – 5.1%, thiazides and furosemide diuretics – 13.2%, statins – 41.2%, and fibrates – 6.6%.As of complications in long-term period after COVID-19, 2.2% had acute kidney failure due to renal artery thrombosis, 11% had thrombotic complications in arteries of upper and lower extremities, 30.9% had signs of vasculitis, 6.6% had myocardial infarctions, 20.6 – stroke.The main hematological tests are provided in table 1.
|
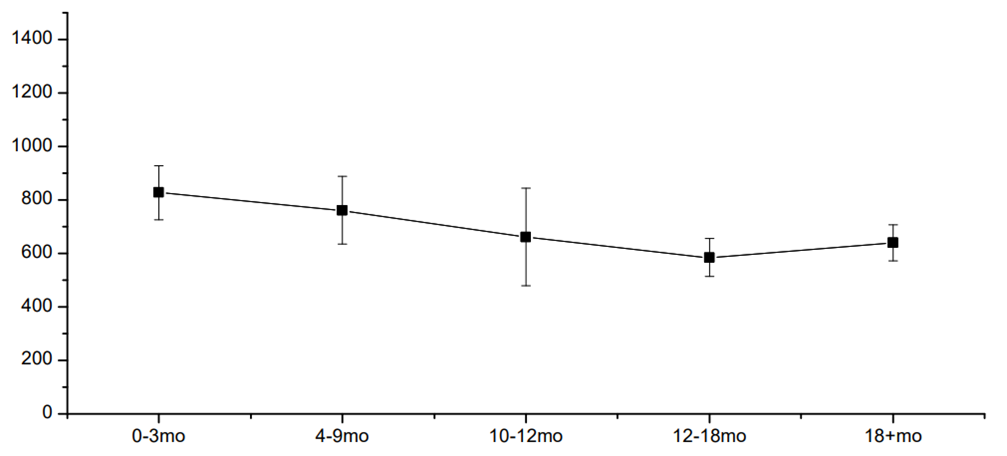 | Figure 1. The mean level of VCAM-1 in 1-24 months after COVID-19 (ng/mL) |
|
4. Discussion
- One of the key pathophysiological pathways in COVID-19 is endothelial dysfunction, which has complex pathogenesis and multiple molecules involved [1]. The increase in the level of VCAM-1 – one of the mediators of vascular inflammation – is a result of reversible activation of vascular endothelial cells within hours or days after the onset of COVID-19 in type II endothelial cells activation. The latter results in procoagulant molecules formation and an increase in the risk of thrombosis. The key message is that this stage (type II activation of endothelial cells) is still reversible compared to further apoptosis and necrosis.In our study, the VCAM-1 level was increased up to 21 months after COVID-19 onset and was not associated with von Willebrand’s factor or D-dimer increase or other disorders in the coagulogram. This means that routine check of coagulogram, D-dimer, and vWF may miss pro-thrombotic changes in microvasculature.Tong M. and coauthors showed a significant increase of endothelial cell adhesion molecules depending on the severity of COVID-19 with a further decrease of its level with convalescence [2]. Vinayagam S., in the review article, clearly showed the benefits of anticoagulant treatment for patients with COVID-19 [5]. The question is how long one should use and which anticoagulant. And another issue is hypocoagulation cases, which, being rare, are of great importance and difficult to manage. Earlier, we have described the case of severe Fisher-Evans’ syndrome in a patient after COVID-19 associated with subcutaneous and submucous hemorrhages and lethal outcomes [6].Salas A. and coauthors, in their study on rats, showed that pre-treatment of heparin did not affect the expression of VCAM-1 in response to induction of inflammation with tumor necrosis factor alpha. Interestingly enough, the authors showed in their work that the level of VCAM-1 was different in different tissues, but its increase was in all tissues (lung, heart, pancreas, stomach, small bowel, caecum, colon, kidney, and muscle), and the level was the highest in lung and kidney followed by heart tissue and pancreas [7]. The latter may be one of the reasons for arrhythmia and diabetes onset after COVID-19.Hippensteel JA and coauthors, in their mini-review, showed the potential role of heparin in the prevention of severe COVID-19: together with anti-coagulant action, heparin is proposed to have an anti-viral effect due to inhibition of the interaction of viral Spike protein with endothelial or epithelial cell surface; and inhibition of inflammatory cells infiltration and dampening of pro-inflammatory signaling. Still, the question of the timeline of heparin use after COVID-19 is open [3].Regarding statins, Qian Y and coauthors showed that simvastatin – compared to other statins – inhibited in vitro activation of endothelial cells triggered by SARS-CoV-2 nucleocapsid protein. The authors also showed that the activation of endothelial cells was only with SARS-CoV-2 but not with the other six types of coronaviruses infecting humans [4].Some authors showed a decrease in VCAM-1 level in patients taking metformin [8,9], DPP4 inhibitors [10,11], and GLP-1ra [12,13]. Regarding SGLT2 inhibitors, there are data showing that this class of glucose-lowering medications does not affect the level of VCAM-1 [14-16]. In our study, we did not see any statistically significant difference in VCAM-1 level depending on glucose-lowering therapy.Strength of the study: this study shows the long-term consequences of COVID-19 on endothelial function for 24 months after acute viral infection. Limitations of the study: patients in our study were not randomized to any group, and the level of VCAM-1 may have been influenced by other factors, so further, more precise studies are needed to prove our findings and also to find the exact mechanisms of potential benefits of the described medicines. Therefore, the external validity of the results should be confirmed in further studies.Also, as we have no data before the infection, the elevated level of VCAM-1 in patients with arterial hypertension who did not take regular antihypertensive therapy may also be related to reasons other than COVID-19.Twenty-two patients who did not know that they had COVID-19 and had not been vaccinated by the time of the study had a high level of neutralizing antibodies (IgG) to COVID-19; thus, the presence of antibodies is an indirect sign of possible COVID-19 before enrolment to the study.
5. Conclusions
- Endothelial dysfunction may be present up to two years after COVID-19, and patients with type 2 diabetes should be monitored closely for post-COVID vascular complications. Use of oral anticoagulants should be considered in type 2 diabetes patients after COVID-19 under the control of hemostasis for at least one year after COVID-19. The potential benefit of glucocorticoids use in terms of endothelial function should be studied more deeply.
ACKNOWLEDGEMENTS
- Authors express their gratitude to the Republican Specialized Scientific-and-Practical Medical Centre of Endocrinology.
Funding/Support
- This study was partly supported by grant A-CC-2021-139 from the Ministry of Innovation of Uzbekistan.
Conflict of Interest
- Authors declare no conflict of interests.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML