-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2023; 13(9): 1250-1254
doi:10.5923/j.ajmms.20231309.16
Received: Sep. 8, 2023; Accepted: Sep. 21, 2023; Published: Sep. 23, 2023

Analysis of the Effect of Hypoglycemic Therapy Preceding COVID-19 on Its Course and Outcomes
Alieva A. V.
Republican Specialized Scientific-and-Practical Medical Centre of Endocrinology Named after Academician Ya.Kh. Turakulov under the Ministry of Health of the Republic of Uzbekistan
Correspondence to: Alieva A. V., Republican Specialized Scientific-and-Practical Medical Centre of Endocrinology Named after Academician Ya.Kh. Turakulov under the Ministry of Health of the Republic of Uzbekistan.
| Email: |  |
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Aim: to study the effect of glucose lowering therapy received by patients with diabetes on the course and outcome of COVID-19. Materials and methods. We analyzed a cohort of patients with type 1 diabetes (213 patients) and type 2 diabetes (763 patients) who had COVID-19 in 2020. Of these, 234 patients (120 men (51.28%) and 114 women (48.72%)) were selected, in whom hypoglycemic therapy did not change for at least 6 months preceding the coronavirus infection. Results. In our study, correlation analysis did not show significant links between the hypoglycemic drugs taken and the course of coronavirus infection. Patients with type 2 diabetes treated with insulin had a high risk of pneumonia (9,656, 95% CI 1,260-73,979). Patients with type 2 diabetes who received insulin or SU were more likely to need hospitalization. The risk of death, the severity of COVID-19 and the need for dexamethasone administration did not depend on the type of hypoglycemic therapy received. Conclusion: treatment of patients with diabetes should be based on the current recommendations, and no matter, what glucose lowering drug is used, achieving glycemic control is key factor to prevent complications.
Keywords: Diabetes, COVID-19, Glucose-lowering therapy
Cite this paper: Alieva A. V., Analysis of the Effect of Hypoglycemic Therapy Preceding COVID-19 on Its Course and Outcomes, American Journal of Medicine and Medical Sciences, Vol. 13 No. 9, 2023, pp. 1250-1254. doi: 10.5923/j.ajmms.20231309.16.
Article Outline
1. Introduction
- Despite the abundance of scientific papers devoted to the peculiarities of the course of coronavirus infection in patients with diabetes mellitus, despite the end of the COVID-19 pandemic, there is still no consensus and uniform standards for hypoglycemic therapy of patients with diabetes in the acute period of coronavirus infection. The majority of recommendations include suspense of any non-insulin drugs in patients with severe infection. While how safe is to prescribe non-insulin glucose lowering agents to patients with non-severe COVID-19 is still a matter of discussion.Taking into account that at the beginning of the COVID-19 pandemic, all recommendations were based on the experience gained during the first pandemic caused by the MERS virus, a detailed analysis of hypoglycemic therapy and its effect on the course of coronavirus infection seems to us an urgent task. So, we aimed to study the effect of glucose lowering therapy received by patients with diabetes on the course and outcome of COVID-19.
2. Materials and Methods
- We performed cross-sectional study, where we analyzed a cohort of patients with type 1 diabetes (213 patients) and type 2 diabetes (763 patients) who had COVID-19 in 2020 in Tashkent. Sampling was full based on the database of COVID-19 patients of Tashkent state Healthcare Department which included all patients who had confirmed COVID-19 since 2020. Of these, data of 234 patients (120 men (51.28%) and 114 women (48.72%)) were selected, in whom hypoglycemic therapy did not change for at least 6 months preceding the coronavirus infection. 22 patients were patients with type 1 diabetes, 181 were patients with type 2 diabetes. Inclusion criteria were: having type 1 or type 2 diabetes diagnosed before 2020, confirmed COVID-19 by rPCR and medical records. Exclusion criteria: changing in glucose-lowering therapy within the last 6 months before the study, pregnancy in 2020 or later. Considering that all patients with type 1 diabetes received insulin, they were included in the subgroups of people who received human or analog insulins for analysis. The average age of patients was 56.89±13.50. The average duration of diabetes was 8.43±5.81 years. The average BMI was 31.64±6.27 kg/m2, while normal BMI was in 8.62% of patients, overweight – 3 15.52%, obesity of the 1st degree – in 58.62%, obesity of the 2nd degree – in 12.07% and obesity of the 3rd degree – in 5.17% of patients.The average blood pressure was 154.60±17.87 / 93.80±10.86 mm Hg. At the same time, 64.96% of patients had hypertension, and 60.68% had ischemic heart disease.14.96% had a mild course of coronavirus infection, 58.97% - moderate-severe, 26.07% - severe. 65.38% were hospitalized in the acute period of coronavirus infection. The average duration of hospitalization during the acute period of COVID-19 was 8.68±1.95 days. 73.93% had pneumonia, 65.81% of patients received dexamethasone. 5 (2.14%) patients died, average age was 58.8±8.93 years. Of the deceased patients, 4 were men, 1 with type 1 diabetes, all with grade 1 obesity, duration of diabetes from 1 to 10 years (average duration of diabetes 6.25 ± 3.30 years), two received metformin (1 – in combination with human insulins, 1 – in combination with analog insulins and iDPP-4), two – SU, one (patient with type 1 diabetes) – analog insulins. All the deceased had a severe course of coronavirus infection with bilateral pneumonia, while only three were hospitalized (the average length of hospital stay was 7.5± 3.32 days), two patients received dexametazone.In total, patients received the following hypoglycemic drugs (as mono- and combination therapy): 44.9% of patients received metformin therapy, although in national standards for the treatment of type 2 diabetes, metformin is a first-line drug and remains as a component of multicomponent therapy throughout the patient's management until eGFR decreases to 30 ml/min/1,73m2 [2; pp.1-132; 22; C. 1-301]. 36.4% of patients received human insulins, 13.7% - analog insulins, 28.2% – sulfonylureas, 8.6% of patients received iDPP-4. Only 1 patient received iSGLT2, so this drug was not included in the subsequent analysis.
3. Results
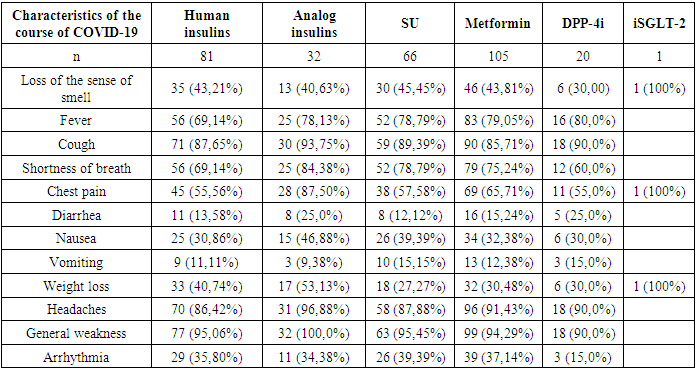
- According to the groups of hypoglycemic drugs, patients did not differ in age or gender, patients taking iDPP-4 were a group of patients with a shorter history of diabetes (compared with patients receiving human insulins), a higher BMI (compared with all other groups), hospitalized for a longer period (compared with patients receiving human insulin). who received SU and human insulins). Patients receiving metformin had a higher BMI (compared with patients receiving insulin) and shorter hospitalization periods in the acute period of coronavirus infection (compared with patients receiving analog insulin).To analyze the effect of hypoglycemic therapy preceding the onset of coronavirus infection on its course and outcomes, we analyzed the frequency of clinical manifestations of COVID-19 depending on the group of hypoglycemic drugs (Table 1).
|
|
|
4. Discussion
- Recommendations for hypoglycemic therapy of patients in the acute period of COVID-19 were mainly in cancellation of oral preparations [1] – metformin due to the risk of lactate acidosis [2-6], iSGLT2 due to the risk of euglycemic ketoacidosis [7, 8]. However, there are data in the literature that indicate the absence of the influence of certain hypoglycemic drugs on the development of adverse outcomes in coronavirus infection in real clinical practice [9,10].A retrospective analysis of 495 patients with type 2 diabetes out of 5473 patients with COVID-19 registered in the database of the insurance system in Korea showed no significant difference in the clinical course and outcomes of coronavirus infection depending on the oral hypoglycemic drugs taken and their combination with insulin [11].These results were confirmed in another study conducted in the same country using multivariate logistic regression [12].The largest-scale study of the outcomes of COVID-19 in diabetes was the CORONADO study, which included 1,317 patients. In this study, there was no effect of hypoglycemic therapy on outcomes (tracheal intubation and death). Metformin therapy before COVID-19 infection was associated with a lower frequency of deaths (HR 0.59; 95% CI 0.42-0.84), however, this advantage was leveled during multivariate analysis [13,14].
5. Conclusions
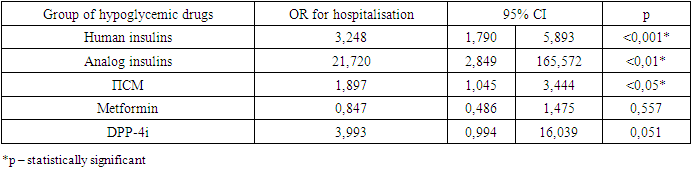
- Thus, in our study, correlation analysis did not show significant links between the hypoglycemic drugs taken and the course of coronavirus infection. Patients with type 2 diabetes treated with insulin had a high risk of pneumonia (9,656, 95% CI 1,260-73,979). Patients with type 2 diabetes who received insulin or SU were more likely to need hospitalization. The risk of death, the severity of COVID-19 and the need for dexamethasone administration did not depend on the type of hypoglycemic therapy received. Treatment of patients with diabetes should be based on the current recommendations, and no matter, what glucose lowering drug is used, achieving glycemic control is key factor to prevent complications.
Limitations
- We had no access to measurements of glycemia in patients with COVID-19 who were not hospitalized during acute infection, so, the impact of glycemic control is not assessed.Strength of the Study: as far as authors are aware, it is the first study performed in the country to assess to role of glucose-lowering therapy in COVID-19 outcomes.
ACKNOWLEDGEMENTS
- Authors express their gratitude to the Tashkent state Healthcare Department, to the Republican Specialized Scientific-and-Practical Medical Centre of Endocrinology, and Tashkent city clinics who worked with patients with acute COVID-19.Competing Interest. Authors declare no conflict of interests.Budget. None.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML