-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2023; 13(9): 1198-1203
doi:10.5923/j.ajmms.20231309.04
Received: Aug. 27, 2023; Accepted: Sep. 12, 2023; Published: Sep. 13, 2023

Study of the Structure and Function of the Intestine in Rats with Chronic Renal Insufficiency
Sh. A. Abdulkhakimov, Khasanova D. A.
Bukhara State Medical Institute, Bukhara, Uzbekistan
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Despite the severe but compensated chronic renal failure that we induced in rats by 9/10 staged nephrectomy, we did not observe corresponding pathological changes in the mucosa and intestines. Even in animals that died prematurely from uremia, there were no signs of erosions, ulcers, or pseudomembranous colitis, which are usually considered characteristic of the uremic condition. Thus, there are significant doubts about the existence of uremic enterocolitis, and these doubts are confirmed after a critical review of the literature on human pathology.
Keywords: Intestinal mucosa, Chronic renal insufficiency, Morphology, Intestinal absorption, Rat
Cite this paper: Sh. A. Abdulkhakimov, Khasanova D. A., Study of the Structure and Function of the Intestine in Rats with Chronic Renal Insufficiency, American Journal of Medicine and Medical Sciences, Vol. 13 No. 9, 2023, pp. 1198-1203. doi: 10.5923/j.ajmms.20231309.04.
1. Introduction
- Earlier literature (Treitz, 1988; Jaffe and Laing, 1994; Mason, 1992) contains extensive statistical data from autopsies documenting pathological changes in the gastrointestinal tract in uremia. These data indicate the presence of edema and hemorrhage in the mucosal and submucosal layers, as well as the presence of single or multiple erosions, ulcers, and pseudomembranous enterocolitis. However, in more recent literature and in light of our current knowledge regarding the various etiologies of pseudomembranous colitis and the results of our own autopsies, serious doubts have emerged regarding the existence of "uremic enterocolitis." These doubts are further supported by conflicting results from animal experiments. While some researchers have observed erosions, ulcers, and uremic enterocolitis (Nairn, Williams, 2015; Carter et al., 2016), others have only noted a slowing of cell regeneration in the mucosa of the small and large intestines without signs of inflammation or epithelial defects (Castrup et al., 2006; et al., 2010; MacDermott et al., 2011, 2014; Lörs and Arnholdt, 2016).In light of this, the aim of our study was to investigate the impact of severe chronic kidney failure on the intestinal mucosa of rats under strictly controlled experimental conditions, using various morphological and functional methods. The significance of the obtained results for human pathology is discussed.
2. Materials and Methods
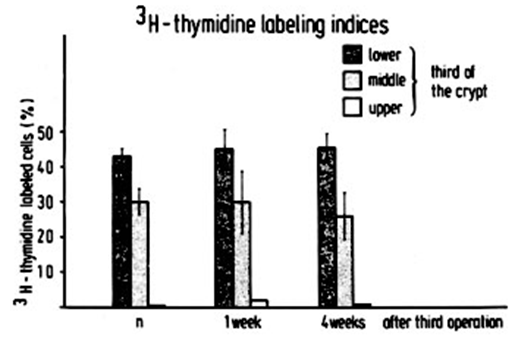
- The experiment utilized 235 female three-month-old Wistar rats weighing between 250 and 280 g.To induce chronic kidney failure, a stepwise nephrectomy procedure was performed following the Morrison modification (1996). Initially, the upper and lower poles of the left kidney were removed (first surgery) after splitting its capsule. Two weeks later, the capsule of the right kidney was split, and complete excision of the right kidney was performed (second surgery). Four weeks later, one-third of the remaining left kidney was resected (third surgery). In the control group, animals underwent three sham surgeries with capsule splitting of both the left and right kidneys. To assess kidney function, periodic measurements of serum creatinine and urea levels were conducted using combined tests for creatinine and urea (Boehringer Company, Mannheim). Blood samples were obtained from the retrobulbar plexus.For the study, the proximal sections of the small intestine, 5 cm distal to the Treitz ligament, and the proximal sections of the large intestine, 5 cm distal to the ileocecal valve, were examined.Optical microscopy. To fix the tissues intraluminally in the intestinal lumen, a 4% buffered formaldehyde solution (pH 7.2) was introduced. Tissue samples were processed, embedded in paraffin, and regular sections were prepared. The sections were then stained with hematoxylin-eosin and periodic acid-Schiff (PAS). Investigations were conducted at 1, 2, 4, 8, and 12 weeks after the third surgery (experimental animals: 20; control animals: 4).Transmission Electron Microscopy. For intraluminal fixation in the intestinal lumen, a 2.5% buffered glutaraldehyde solution (pH 7.2) was introduced. After tissue samples were extracted, they underwent subsequent fixation in a 1% buffered osmium tetroxide solution (pH 7.2), embedding in epoxy resin, and preparation of 2 mm semithin sections. The sections were stained with methylene blue, PAS, and special silver techniques. Thin sections were obtained using the OmU2 ultramicrotome (Reichert) and contrasted with uranyl acetate and lead citrate. Electron photomicrographs were taken using an EM 301 electron microscope (Philips). Investigations were conducted at 1, 2, 4, and 8 weeks after the third surgery (experimental animals: 16; control animals: 4).Morphometry. Semithin sections of the duodenum and colon were measured using a semi-automatic image analyzer (MOP/AMO 1 (Kontron)). The following parameters were examined: the number of villi and crypts per mm of mucosal length; height, width (at the midpoint of the villi), and circumference of the villi; total number of villous cells and goblet cells; height of enterocytes (at the midpoint of the villi); depth, width, and circumference of the crypts. For each animal, ten randomly selected areas of the mucosa were analyzed. Investigations were conducted at 1, 2, 4, and 8 weeks after the third surgery (experimental animals: 16; control animals: 4).Authoradiography. Animals were intrabdominally injected with 3H-thymidine (dosage: 500 pCi, specific activity: 20.0 Ci/mmol) one hour prior to euthanasia. Tissue samples were fixed in a 4% formaldehyde solution with the addition of non-radioactive thymidine (0.5 mg/mL). Paraffin sections with a thickness of 4 μm were coated with a layer of photoemulsion (Ilford L4 Nuclear Research Emulsion in gel form, immersion method), exposed for 21 days, developed, and stained with hematoxylin-eosin. The determination of the 3H-thymidine labeling index was performed by counting 1000 epithelial cells in the upper, middle, and lower thirds of the crypts of the duodenum and colon. The studies were conducted 1 and 4 weeks after the third operation (experimental animals: 10; control animals: 5).Histoenzymology. The following enzymes were investigated: alkaline phosphatase (using sodium β-naphthyl phosphate as a substrate and fast blue B as a dye, pH 9.2), acid phosphatase (using sodium β-glycerophosphate as a substrate and lead salt, pH 5.0), nonspecific esterase (using α-naphthyl acetate as a substrate and hexazonium pararosaniline as a dye, pH 7.4), and succinate dehydrogenase (using sodium succinate as a substrate and nitroblue tetrazolium as a dye, pH 7.6). The studies were conducted 1 and 4 weeks after the third operation (experimental animals: 10; control animals: 5).Absorption studies. The net rates of water, glucose, and electrolyte absorption were determined. After suturing the 10-cm proximal loop of the duodenum into the intestinal cavity, 2 mL of a standard solution (concentration in mmol/L: NaCl: 105; NaHCO3: 25; KCl: 4.5; glucose: 11.2) was introduced. After 1 hour, the looped segment was removed, and the fluid in the lumen was analyzed. Net water movement was determined by measuring the change in concentration of C14-polyethylene glycol, which served as a volume marker. Net rates of glucose and electrolyte transport were calculated based on the difference in their concentrations at the beginning and end of the experiment, with known net fluid movement. The net transport rates are indicated in μL of water or μmol.
3. Results
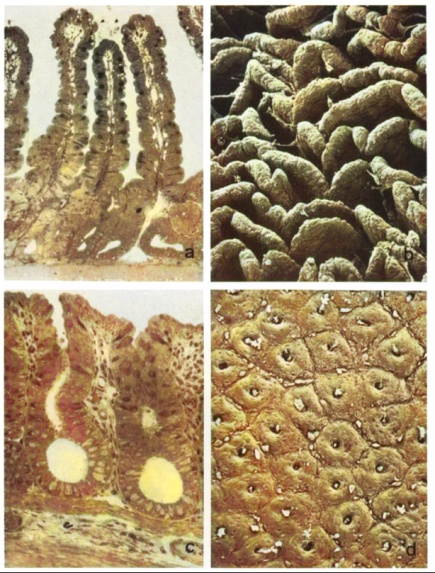
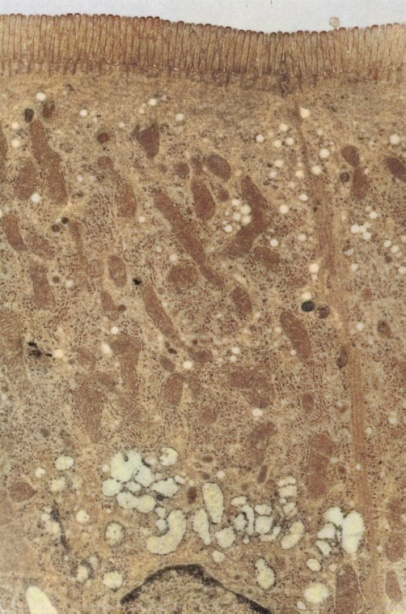
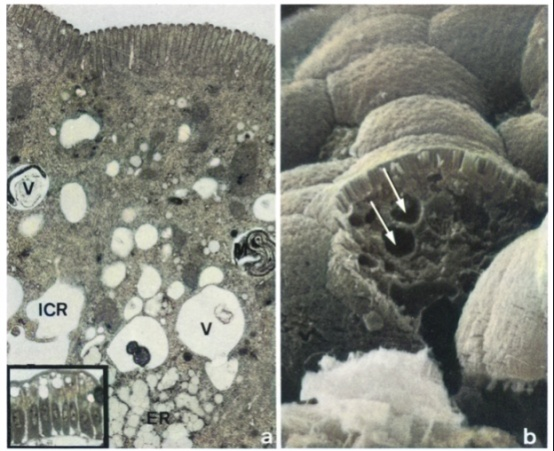
- Mortality. Approximately 70% of all experimental animals that underwent 9/10 nephrectomy died. 15% of experimental animals died after the first operation, 17% after the second operation, and 35% after the third operation. The high mortality rate was due to anesthetic incidents, postoperative bleeding and infections, as well as death caused by uremia resulting from excessive resection of kidney parenchyma.Animal weight. Surviving animals temporarily lost 5% of their weight after the first and second operations, and lost 15% to 25% of their weight after the third operation. However, by the end of each first operative week, the animals started to regain weight, and by the end of the experimental period, they returned to their initial weights. Animals subjected to sham surgery only lost 5% of their weight after each operation. At the time of the studies, the control animals weighed 25% more than the experimental animals.After the first operation, the serum levels of creatinine and urea increased on average to 0.71 mg% and 47 mg%, respectively (normal levels: serum creatinine 0.5 mg%, serum urea 38 mg%). During the first week after the second operation, the serum levels of creatinine and urea increased to values exceeding the normal range by 3.5 times (1.75 mg% and 137 mg%), while in the fourth week, they were only 2.5-3 times higher than normal (1.38 mg% and 125 mg%). In the first week after the third operation, the serum levels of creatinine and urea were 5-7 times higher than normal (2.52 mg% and 256 mg%). Subsequently, the levels of creatinine and urea in the serum decreased and remained constant at values exceeding the normal range by 3.5-4.5 times (1.75-2.2 mg% and 175-186 mg%) respectively.Morphological results. Macroscopically, the mucous membrane of the small and large intestine did not differ between experimental and control animals. It had a smooth velvety structure and a pale red color in both groups. No erosions or ulcers were found. Under light microscopy, no differences were observed between experimental and control animals. The mucous membrane of the small intestine (Figure 1a) maintains a regular structure with evenly distributed thin villi and tightly packed crypts. The enterocytes exhibit columnar shape and have a distinct microvillus border on semi-thin sections.Occasionally, some enterocytes located in the upper and middle thirds of the villi show a sporadic occurrence of cytoplasmic vacuoles of varying sizes, which is considered an unstable phenomenon (Figure 3a, inset). The mucous membrane of the large intestine (Figure 1c) also displays an intact structure with epithelial cells showing no signs of cellular damage. No epithelial defects are visible in either the small or large intestine under light microscopy. The lamina propria in both the small and large intestine appears narrow and shows no signs of inflammation. There are no pathological changes observed in the submucosa and its vessels, as well as in the smooth muscular layer.Scanning electron microscopy confirms the results of light microscopy. The small intestine exhibits regularly arranged leaf-like and densely packed villi (Figure 1b).
4. Discussion
- To conduct this study, it was necessary to develop an appropriate method for inducing chronic kidney insufficiency that would result in a significant elevation of creatinine and urea levels while providing a sufficiently long survival time. Nephrotoxic agents such as uranium nitrate (Nomiyama et al., 2008; Fukuda and Kopple, 2010) could not be used due to their potential direct toxic effects on the intestinal mucosa. After numerous preliminary experiments, a modified gradual 9/10 nephrectomy technique, based on the methods described by Chanutin and Ferris (1992), Morrison (1996), and Heitz et al. (1994), was chosen as the most suitable method for inducing severe but compensated chronic kidney insufficiency. While a 5/6 nephrectomy ultimately leads to a two to threefold increase in serum creatinine and urea levels, the 9/10 nephrectomy results in a four to fivefold increase. The high number of animals dying from uremia after the 9/10 nephrectomy, despite extended adaptation periods between surgeries, indicates that this method achieved the upper limit of still-compensated kidney insufficiency with a sufficiently long survival time. Despite the presence of severe chronic kidney insufficiency, no significant pathological changes were observed in the mucous membranes of the small and large intestines, despite the use of various methods to detect them. Specifically, no erosions, ulcers, or pseudomembranous enterocolitis, which have been described as characteristic features of uremic conditions in earlier publications (Jaffe and Laing, 2004; Mason, 2012; Carter et al., 2016), were detected. This finding also applies to animals dying prematurely from uremia. Even with the use of electron microscopy, no changes indicating toxic damage to the mucosal epithelial cells or impairment of their differentiation were detected. Additional evidence of the absence of uremia-related pathological changes in the mucous membranes was obtained through morphometric and autoradiographic studies. Therefore, no statistically significant differences were found in the absorptive activity of the intestinal mucosa between rats with chronic kidney insufficiency and control animals. The vacuoles found in some enterocytes, which could be identified as autophagic vacuoles using electron microscopy, are highly variable and not specific to uremic damage to the intestinal mucosa. On the contrary, these vacuoles can be found even under physiological conditions in the extrusion zone of the villus tip. In animals with chronic kidney insufficiency, these vacuoles are also only found in close proximity to the extrusion zone and never near the base of the villus or in crypt epithelium. Therefore, they should be regarded as expressions of nonspecific degenerative changes rather than generalized toxic (uremic) cell damage.Considerable difficulties arise when comparing our morphological and functional data with those described in recent publications (human biopsies: Riecken et al. 2009; Goldstein et al. 2011; animal experiments: Castrup et al. 2010; Grimmel et al. 2011; L6hrs and Arnholdt 2006; Wizemann et al. 2008; McVicar et al. 2010; Sterner et al. 2010) due to the different methods used to assess changes and the varying severity of chronic renal failure. However, all studies, including our own, share a common feature: the absence of erosions, ulcers, or pseudomembranous enterocolitis. Furthermore, there is no evidence that uremic toxins such as urea, various guanidine derivatives, aromatic derivatives, or aliphatic amines (Bergstr6m and Bittar 1996; Dobbelstein 2001; SetSAfi 2008) have detrimental effects on the intestinal mucosa. In particular, urea, often considered the main uremic toxin, does not appear to negatively affect the intestinal mucosa, as demonstrated by Bolleman and Mann (2007) and Kuchera and Malinsky (2004), who implanted ureters into the intestinal wall. Despite the high local concentration of urea in these intestinal segments, no erosions, ulcers, or pseudomembranous enterocolitis were observed in experimental animals or humans. Therefore, there are serious doubts about the existence of uremic enterocolitis described in previous publications (Jaffe and Laing 2014; Mason 2012) and also in more recent pathology textbooks (Eder and Gedigk 2014; Sleisinger and Fordtran 2013; Robbins 2014). It is now known that pseudomembranous enterocolitis does not represent a single etiological disease and can be caused by various factors such as shock, postoperative circulatory disturbances, antibiotic therapy, or mercury poisoning. Birnbaum (2011), analyzing the etiology of 40 cases of pseudomembranous enterocolitis, pointed out that in all cases with uremia, there was another etiological factor that could independently cause pseudomembranous enterocolitis. Thus, he believed that the association between pseudomembranous enterocolitis and uremia was coincidental. This conclusion is supported by a study of 265 pathological cases of chronic uremia conducted by Mason (2012). He found that pseudomembranous enterocolitis manifested in only 19.6% of all patients with uremia but also in 10% of all patients without uremia.
5. Conclusions
- Certainly, the results of our systematic experimental studies cannot be directly extrapolated to human pathology. However, in conjunction with the results and interpretations of other researchers mentioned above, our findings strongly indicate that uremia itself does not play a role in the etiology of pseudomembranous enterocolitis. Furthermore, the preservation of intestinal morphology underscores the importance of this model for studies focusing on renal pathophysiology without compromising the integrity of the gastrointestinal tract. These findings contribute to the body of knowledge in this field and provide a foundation for future research endeavors.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML