-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2023; 13(8): 1178-1182
doi:10.5923/j.ajmms.20231308.31
Received: Aug. 9, 2023; Accepted: Aug. 25, 2023; Published: Aug. 28, 2023

Peculiarities of Incidence and Contribution of the Polymorphous Estrogen Receptor Gene ESR1 (rs9340799) in Various Forms of Genital Prolapse
Nasimova Nigina Rustamovna
Samarkand State Medical University, Uzbekistan
Correspondence to: Nasimova Nigina Rustamovna, Samarkand State Medical University, Uzbekistan.
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

We have studied the occurrence and contribution of the polymorphic estrogen receptor gene ESR1 (rs9340799) among women with genital prolapse. Gene evaluation was performed by analyzing DNA samples by standard PCR. Our results of the study allowed us to establish that the mutant A allele and the A/A genotype of the rs9340799 polymorphism in the estrogen receptor gene ESR1 are associated with an increased risk of developing a severe degree of genital prolapse and can be considered as independent genetic markers that have a pathogenetic contribution to the aggravation of the disease, while the main genotype G/G is a protective marker that prevents the transition of the disease into a severe form.
Keywords: Genital prolapse, Estrogen receptor, Polymorphism, Gene, ESR1 (rs9340799), Frequency, Allele, Genotype, Carriers
Cite this paper: Nasimova Nigina Rustamovna, Peculiarities of Incidence and Contribution of the Polymorphous Estrogen Receptor Gene ESR1 (rs9340799) in Various Forms of Genital Prolapse, American Journal of Medicine and Medical Sciences, Vol. 13 No. 8, 2023, pp. 1178-1182. doi: 10.5923/j.ajmms.20231308.31.
1. Introduction
- Genital prolapse (PG) today is a very common gynecological pathology, in which approximately 37% of women, due to the aggravation of their general condition and deterioration in the quality of life, turn to medical institutions for surgical treatment [6,10].It is known that the normal function of the pelvic floor is maintained due to the full interaction of the bones, muscles, ligaments and fascia of the pelvis, as well as their innervation. [1,4]. Their various defects arising under the influence of certain factors (age, menopause, obesity, genetic status, bad habits, hard physical work, physical inactivity, chronic constipation, connective tissue pathology, a history of operations) undoubtedly serve as the basis for the development of PG in women [3]. Meanwhile, it is not always possible to determine the exact pathogenetic mechanisms of the formation of this complex pathology, which are based on very unclear complex processes. In this regard, the increasing interest of modern researchers is attracted by more subtle mechanisms for the development of PH, which involve the participation of a number of polymorphic genes, the dysregulation of which leads to structural and quantitative changes in the components (collagen, fibronectin, elastin, etc.) of the pelvic floor tissues [7,8,9].Among the existing scientific data on molecular genetic factors, the literature presents the results of studies aimed at studying the contribution to the development of PG of the polymorphic estrogen receptor gene (ESR1) [2]. However, along with studies proving the participation of the above genetic variants of polymorphisms in the pathogenesis of PG, there are also conflicting data regarding their influence on the development of changes in the structure and content of the constituent tissues of the pelvic floor, which serve as the basis for the development of PG. [5].Taking into account the existing controversial discussions in this area, it seemed to us very important to study the presence of an associative relationship between the polymorphic ESR1 gene (rs9340799) and the risk of developing various forms of PG.
2. Material and Methods
- The study was conducted with the participation of 171 women (median age 25±68 years) living in the Republic of Uzbekistan. Among the entire selected cohort, 84 women (the 1st - the main group of patients) were diagnosed with genital prolapse, verified as a result of a survey in the maternity complex No. 3 and the private clinic "Doctor Shifo Bakht" (Samarkand) from 2018 to 2023. G. The remaining 87 women were healthy (5th healthy control group). The main group of women with genital prolapse, depending on the severity, was divided into three groups: 2nd (n=28) with mild severity, 3rd (n=39) with moderate severity and 4th (n=17) with a history of severe disease severity, of comparable age and sex in the general group of RA patients. Molecular genetic studies were performed on the basis of the laboratory of medical genetics of the Republican Specialized Scientific and Practical Medical Center for Hematology (Republic of Uzbekistan, Tashkent). In accordance with the generally accepted method, DNA was isolated from blood leukocytes. At the same time, using the Applied Biosystems 2720 system (USA), an analysis (SNP-PCR) of the ESR1 gene (rs9340799) was carried out using the Litekh test systems (Russia). Mathematical analysis of the results was carried out using the program "Open Epi 2009, Version 9.3".
3. Results and Discussion
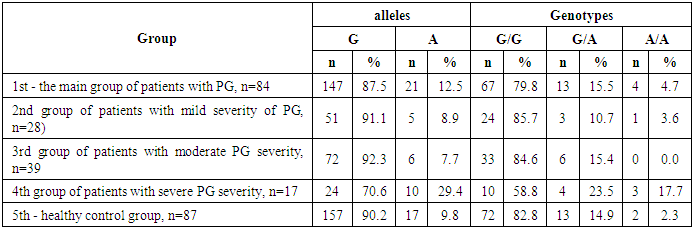
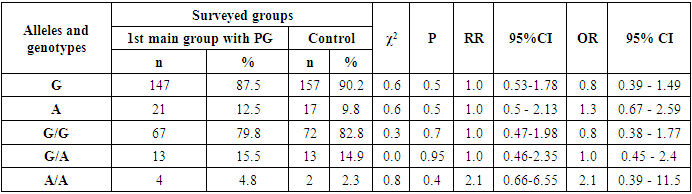
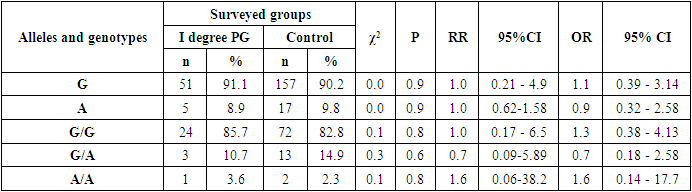
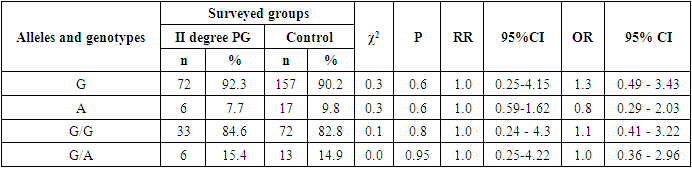
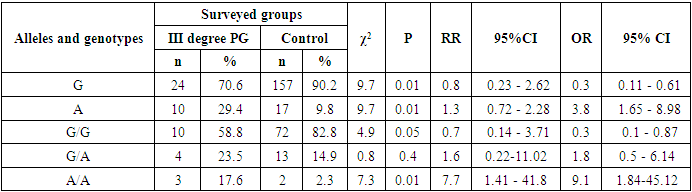
- Studying the correspondence between the distribution of observed (Ho) genotypic frequencies (G/G, G/A and A/A) of the polymorphic estrogen receptor gene ESR1 (rs9340799) to their expected (He) frequencies according to Hardy-Weinberg equilibrium (HWB) in groups of patients with PH (n=84), we found a slightly deviated RCM (p<0.05). This was due to some excess in the observed frequency of the attenuated A/A mutant genotype (χ2=5.5; P=0.003; df=1). Assessing the nature of the distribution of frequencies of alleles and genotypes for the polymorphic gene of the estrogen receptor ESR1 (rs9340799) among control healthy women (n=87), the proportions of the main G and mutant A alleles were determined in 90.2%/157 and 9.8%/17 cases, respectively, with the detection of carriage by all three possible variants of the genotypes G/G, G/A and A/A, respectively, in 82.8%/72; 14.9%/13 and 2.3%/2 cases (Table 1). Meanwhile, in the 1st main group (n=84) of women with PG compared with healthy ones, the frequency of the main G allele slightly decreased from 90.2% / 157 to 87.5% / 147, and the weakened A allele, on the contrary, increased from 9.8% / 17 to 12.5%/21. Naturally, with allelic frequency compared with healthy, the proportion of the main G/G genotype decreased from 82.8%/72 to 79.8%/67, with an increase in the frequencies of heterozygous (G/A: from 14.9%/13 to 15.5%/13) and mutant (A /A: from 2.3%/2 to 4.7%/4) genotypes. Next, we evaluated the features of the frequency distribution of allelic and genotypic variants of the polymorphism of the estrogen receptor gene ESR1 (rs9340799) among women with PH, depending on the severity of the pathology. Thus, compared with healthy controls in the 2nd group of patients with mild PH severity (n=28), the frequency of the main allele G slightly increased to 91.1%/51 cases with a decrease in the weakened form (A) to 8.9%/5. Accordingly, with these features, the proportion of the main G/G increased to 85.7%/24, with a decrease in the frequency of the heterozygous variant (G/A) to 10.7%/3 and an increase in the proportion of the weakened mutant form of the A/A genotype to 3.6%/1. In parallel, the analysis of the distribution of allelic and genotypic frequencies in the 3rd group of women with PG with moderate severity (n=39) compared with the healthy group showed a similar picture observed in the 2nd group. In particular, an increase in the shares of the main allele G to 92.3%/72 and the G/G genotype to 82.8%/72 was found, while a decrease in the frequency of the weakened A genotype to 7.7%/6 and an increase in the proportion of the heterozygous form of the G/A genotype to 15.4%/ 6 cases. Here it is also important to note the complete absence of cases of carriage of the weakened A/A mutant genotypes among women with moderate severity of PG. Studying the nature of the distribution of frequencies of alleles and genotypes according to the polymorphism of the estrogen receptor gene ESR1 (rs9340799) in the 4th group of patients with severe PH (n=17), compared with healthy women, a marked decrease in the frequencies of the main allele G was found to 70.6%/24 vs. 90.2%/157 and G/G genotype up to 58.8%/10 versus 82.8%/72, respectively. This pattern was accompanied by a pronounced increase in the frequencies of the weakened form of the A allele up to 29.4%/10 vs. 9.8%/17, the heterozygous G/A genotype up to 23.5%/4 vs. 14.9%/13, and the mutant A/A genotype up to 17.7%/3 vs. 2.3% /2 (Table 1).
|
|
|
|
4. Conclusions
- A multilateral analysis to study the characteristics of the distribution and the level of differences in the distribution of allele frequencies and genotypes of the rs9340799 polymorphism in the estrogen receptor gene ESR1 among women with various forms of genital prolapse with pelvic floor insufficiency and healthy women made it possible to establish the presence of a statistically significant increase in the risk of developing a severe form of the disease among carriers weakened mutant allele A and genotype A/A. In particular, compared with healthy women among carriers of the A allele and A/A genotype, the risk of developing a severe form of PH is statistically significantly increased by 3.8 (χ2=9.7; P=0.01) and 9.1 (χ2=7.3; P=0.01) times, respectively, whereas, compared with women with mild and moderate PH severity, in carriers of the A allele, the risk of developing a severe degree is statistically significantly increased by 4.3 (χ2=6.4; P=0.03) and 5.0 (χ2=9.1; P=0.01) times, respectively, despite the fact that among carriers of the main G/G genotype, on the contrary, due to its protective effect, the risk of transition to a severe degree with mild and moderate severity is statistically significantly reduced by 4.2 times (χ2=4.1; P=0.05) and 3.9 (84.6% vs. 58.8%; χ2=4.4; P=0.05). Therefore, it can be concluded from this that the mutant allele A and the A/A genotype of the rs9340799 polymorphism in the estrogen receptor ESR1 gene are associated with an increased risk of developing a severe degree of genital prolapse and can be considered as independent genetic markers that have a pathogenetic contribution to the aggravation of the disease, while the main genotype G/G is a protective marker that prevents the transition of the disease into a severe form.
References
| [1] | Akın Y, Young M, Elmussareh M, Charalampogiannis N, Gözen AS. The novel and minimally invasive treatment modalities for female pelvic floor muscle dysfunction; beyond the traditional. Balkan Med J. 2018; 35: 358–66. |
| [2] | Allen-Brady K, Chua JWF, Cuffolo R, Koch M, Sorrentino F, Cartwright R. Systematic review and meta-analysis of genetic association studies of pelvic organ prolapse. Int Urogynecol J. 2022; 33: 67–82. |
| [3] | Akhmedov F.K. biochemical markers of preeclampsia development and criteria for early diagnosis- Art of Medicine. International Medical Scientific Journal, 2022. 10.5281/zenodo.6635595. |
| [4] | Deng Z-M, Dai F-F, Yuan M-Q, Yang D-Y, Zheng Y-J, Cheng Y-X. Advances in molecular mechanisms of pelvic organ prolapse (Review). Exp Ther Med. 2021; 22: 1009. |
| [5] | Huang L, Zhao Z, Wen J, Ling W, Miao Y, Wu J. Cellular senescence: a pathogenic mechanism of pelvic organ prolapse (Review). Mol Med Rep. 2020; 22: 2155–62. |
| [6] | Isali I., Abdeldayem J., El-Nashar S. Gene expression in urinary incontinence and pelvic organ prolapse: a review of literature // Current Opinion in Obstetrics and Gynecology. – 2020. – Т. 32. – №. 6. – С. 441-448. |
| [7] | Jokhio AH, Rizvi RM, MacArthur C. Prevalence of pelvic organ prolapse in women, associated factors and impact on quality of life in rural Pakistan: population-based study. BMC Womens Health. 2020; 20:1–8. |
| [8] | Nakad B. Et al. Estrogen receptor and laminin genetic polymorphism among women with pelvic organ prolapse //Taiwanese Journal of Obstetrics and Gynecology. – 2017. – Т. 56. – №. 6. – С. 750-754. |
| [9] | F.K. Akhmedov. The role of interleukin 10 in the development of preeclampsia: diagnosis and prognosis- British Medical Journal, 2022 Volume-2, No 410.5281/zenodo.6912557. |
| [10] | F.K. Akhmedov., M.N. Negmatullaeva. The significance of genetic factors and new aspects in predicting preeclampsia (overview)- Thematic journal of microbiology, 2021. 10.5281/zenodo.5081885. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML