-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2023; 13(8): 1175-1177
doi:10.5923/j.ajmms.20231308.30
Received: Aug. 5, 2023; Accepted: Aug. 22, 2023; Published: Aug. 28, 2023

Effectiveness of IL-17 Inhibitors in Reactive Arthritis
Kh. S. Axmedov1, F. I. Khalmetova2
1Professor, Tashkent Medical Academy, Uzbekistan
2Senior Lecturer, Tashkent Medical Academy, Uzbekistan
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

According to the literature, a small number of observations does not yet allow an objective assessment of the use of tumor necrosis factor-α inhibitors in treatment-resistant reactive arthritis. Genetic studies of G197A polymorphism of the IL 17A gene in patients with reactive arthritis revealed their relationship with resistance to IL-17 inhibitors, as well as disease activity during treatment with this medicine.
Keywords: Reactive arthritis, Joints syndrome, IL-17 inhibitors, Genotypes, Spondyloarthritis
Cite this paper: Kh. S. Axmedov, F. I. Khalmetova, Effectiveness of IL-17 Inhibitors in Reactive Arthritis, American Journal of Medicine and Medical Sciences, Vol. 13 No. 8, 2023, pp. 1175-1177. doi: 10.5923/j.ajmms.20231308.30.
Article Outline
1. Introduction
- Treatment of patients with reactive arthritis (ReA) remains one of the most difficult problems of modern rheumatology. Its relevance is due to the progressive course of the disease, the severity of the lesion of the musculoskeletal system, the high incidence of lesions in people of working age, the early decline in functional abilities, the loss of professional and social skills, the difficulty of physical and psychological adaptation of patients to impaired motor functions, significant disability, which represent a serious general medical and social problem, leading to huge economic losses.Currently, significant therapeutic progress has been made due to the advent of biological drugs, which have had a huge impact on the effectiveness of therapy and significantly improved the course of autoimmune processes in many patients with rheumatological pathologies. According to the literature, a small number of observations does not yet allow an objective assessment of the use of tumor necrosis factor-α inhibitors in treatment-resistant ReA. But in the clinical case of Tanaka et al., antibodies to the interleukin-6 receptor were used and effectively progressed the improvement of the symptoms of ReA, which does not work on standard protocols. According to a study by Mens et al. treatment of ReA, which was complicated with spondyloarthritis, drugs with IL-17 inhibitors had a positive effect on the course of the disease.The aim of our study was to investigate the relationship between the G197A polymorphism of the IL 17 A gene and the presence of resistance to secukinumab in ReA patients with spondyloarthritis.
2. Materials and Research Methods
- The study was conducted in the third city clinical hospital №3 in the department of rheumatology of the city of Tashkent. The study was conducted in 76 patients (73 women, 3 men, 24-65 years) of ReA with spondyloarthritis. The control group consisted of 24 healthy volunteers without a burdened rheumatological history. ReA was diagnosed according to the American College of Rheumatology (ACR) criteria and disease activity was calculated using the DAS28 calculator. All patients who participated in our study were prescribed basic therapy with the IL-17A inhibitor secukinumab (at a dose of 150 mg subcutaneously) and the patients were followed up for 6 months.Static processing of the data obtained during the study was carried out using the computer program EXCEL and STATISTICA 6.0. To identify the correspondence of genotype distributions to the expected values at Hardy-Weinberg equilibrium and to compare the distributions of genotype and allele frequencies in two subpopulations, the χ2 test (Pearson) was used, where p<0.05 was considered statistically significant. The limits of the 95% confidence interval (CI) were calculated by the method of B. Woolf.Genomic DNA was extracted from whole blood collected in EDTA tubes using the GeneMATRIX Quick Blood DNA Purification Kit (Poland). Identification of all studied polymorphisms in the IL17A gene was performed using the TaqMannSNP genotyping test: 17A (IL-17A G-197A). The reaction was carried out in duplicate using the Fast Real-Time PCR Detection System (Germany).
3. Results and Discussion
- In our study, 76 patients with ReA and 24 healthy volunteers without a rheumatological history were studied. All patients underwent genotyping of the G197A polymorphism of the IL 17A gene. The results of genotyping are shown in Picture 1.
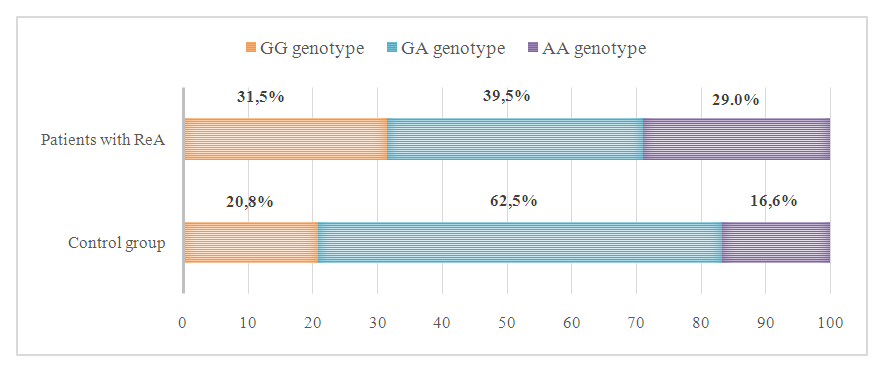 | Picture 1. Percentage distribution of genotypes of G197A polymorphism of the IL 17A gene in ReA patients with spondyloarthritis and in the control group |
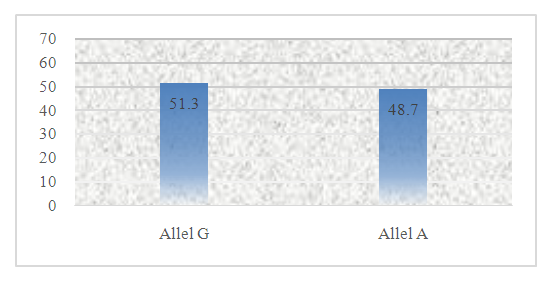 | Picture 2. Distribution of alleles of polymorphism G197A IL 17A gene |
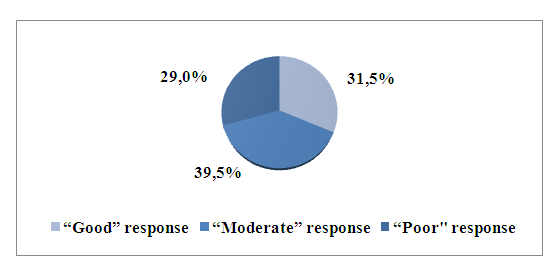 | Picture 3. Percentage distribution of ReA patients by medication response to secukinumab treatment |
4. Conclusions
- Genetic studies of G197A polymorphism of the IL 17A gene in patients with ReA revealed their relationship with resistance to secukinumab, as well as disease activity during treatment with this drug. ReA patients with the GG genotype have a poor response (resistance) to secukinumab treatment, as a result, the disease proceeds with a high degree of disease activity, compared with patients with GA and AA genotypes. Patient genotyping can be used to determine the effectiveness of secukinumab drug therapy and personalized selection of treatment methods for patients with ReA.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML