Arifov S. S.1, Nazarov J. I.2, Axmadxojayev D. I.2
1Center for the Development of Professional Qualification of Medical Workers, Uzbekistan
2Fergana Medical Institute of Public Health, Uzbekistan
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
Allergic diseases represent a major public health problem worldwide. The frequency of allergic rhinitis in the general population is 10-20%, 68% of which is seasonal allergic rhinitis. In 57.14% of patients with seasonal allergic rhinitis, initial vagotonia combined with predominantly over-provisioning activity of the hyperdiastolic type was found. According to the severity of the course of exacerbation of seasonal allergic rhinitis 18,25% had mild course, 57,94% - medium severity of course and 23,81% - severe course of the disease. Application of corrective vegetotropic therapy allows to increase the effectiveness of treatment of seasonal allergic rhinitis exacerbation in vagotonics (57,14% ) in 1,21 times in terms of total clinical effectiveness and in 1,64 times in terms of clinical recovery of patients with seasonal allergic rhinitis. The average increase in the effectiveness of complex treatment of seasonal allergic rhinitis including vagotonia correction is 1.42 times.
Keywords:
Seasonal allergic rhinitis, Severity of course, State of autonomic nervous system, Complex treatment
Cite this paper: Arifov S. S., Nazarov J. I., Axmadxojayev D. I., The Condition of the Vegetative Nervous System and Methods of Correction of Its Changes in Patients with Seasonal Allergic Rhinitis, American Journal of Medicine and Medical Sciences, Vol. 13 No. 8, 2023, pp. 1168-1174. doi: 10.5923/j.ajmms.20231308.29.
1. Introduction
Allergic rhinitis (AR) is a fairly common pathology of the body, in which the target organ is the nasal cavity, characterized by a symptom complex that includes swelling of the mucous membrane and nasal congestion, itching in the nasal cavity and sneezing, rhinorrhea and difficulty in nasal breathing [8,12,15]. Although AR is not life-threatening, it sharply reduces the quality of life, physical and social activity of the patient, it can turn into bronchial asthma, which affects from 20 to 60% of the population [9,13,14].It is not in vain that AR was called the “epidemic of the 20th century”, the increase in the incidence of this pathology cannot be only an increase in the number of seekers and an improvement in diagnostics, which means that its increase is real evidence of an increase in the sensitization of the population of the whole world [17].One of the main adaptive systems of the body is the autonomic nervous system (ANS), which provides the connection of the body with the environment and internal environment, regulating the metabolism and functions of organs and tissues in accordance with changes in this environment, along with the integration of all organs into a single whole [2,18].The ANS consists of two divisions: suprasegmental and segmental. Segmental can be conditionally considered a peripheral part of the ANS, suprasegmental - central [5]. The suprasegmental level of the ANS includes the basics of coordination, regulation of homeostasis, reactions to external and internal changes [2]. At the segmental level, the sympathetic and parasympathetic divisions are divided. With an increase in the tone of the sympathetic department, energy resources are mobilized, the body's readiness to respond to external stimuli is formed, while the activation of its parasympathetic department leads to the accumulation of energy resources and the intensification of plastic reactions [1,6].Severe disturbances in the functional activity of the ANS - autonomic hyperreactivity, are observed in most patients with polypous rhinosinusitis [7,10].There are data on the study of the state of the ANS in AR in children [1,3,16]. Daliev A.G. cites data that among children with AR, persons with the vagotonic type of IWT significantly prevailed (53.9%), in children with the vagotonic type of IWT, AR was often characterized by a more severe course - a moderate course was detected in 33.1.6%, severe - 13.36%, while these figures were 11.98% and 5.99% for normotonia, 11.98% and 5.53%, respectively, for sympathicotonia [4].The imbalance of adaptive mechanisms primarily affects macro- and microhemodynamics, which cannot but affect the pathogenesis of allergic inflammation [22].Considering that vegetative dysfunctions accompany most pathological processes in the body, the identification of vegetative disorders and clarification of their nature is essential both for diagnosing the disease and for choosing therapeutic agents [20,21].Thus, there is no doubt about the importance of the state of the ANS in the pathogenesis of SAD, which requires further study of the state of the ANS and the clinical symptoms of autonomic imbalance, which means that further studies of the autonomic predominance of patients with SAD are more relevant and in demand than ever.This was the stimulus for the present study, the purpose of which was to study the state of the autonomic nervous system in patients with seasonal allergic rhinitis and the effectiveness of corrective treatment of autonomic innervation disorders in the complex treatment of seasonal allergic rhinitis.
2. Material and Research Methods
Us for the period 2020–2023. 126 patients with SAD aged 21–50 years (mean age 31.6±1.4 years) were examined, of which 52 were men and 74 were women. The average age of the observed men was 33.7±1.3 years; 29.2±1.5 years. The control group (CG) consisted of 32 practically healthy individuals, the average age of which was determined as 31.3±1.6 years, without nasal breathing disorders and changes in the nasal cavity and nasopharynx during rhinoscopy.When making a diagnosis, the ICD 10 classification was followed (class X - "Diseases of the respiratory system", section "Other diseases of the upper respiratory tract" (J30-J39), J30.1 - Allergic rhinitis caused by plant pollen, J30.2 - Other seasonal allergic rhinitis).The development included patients who at the time of the study had eosinophilia and IgE in peripheral blood and smears from the nasal cavity.We applied clinical, laboratory, functional research methods, as well as statistical processing of the research results.According to the visual analogue scale (VAS), the studied patients subjectively determined the severity of SAD.Assessment of the state of the ANS was carried out according to the characteristics of the initial vegetative tone (IVT), vegetative reactivity and vegetative support of activity (VAS). The vegetative tone and vegetative reactivity make it possible to judge the homeostatic capabilities of the body, the VOD - its adaptive mechanisms. IWT was evaluated according to the results of the standardized “Questionnaire for identifying signs of vegetative changes” by Guillaume-Vein and “Research scheme for detecting signs of autonomic disorders”, developed by the Russian Scientific and Methodological Center for Autonomic Pathology under the direction of A.M. Wayne [2]. The “Questionnaire for identifying signs of autonomic changes” was filled in by the subject, and the “Research scheme for detecting signs of autonomic disorders” was filled in by the doctor.Vegetative reactivity was assessed by the Dagnini–Ashner ocular reflex by recording the heart rate (HR) for 30 seconds. To determine the adaptive-compensatory capabilities of the organism, we conducted an orthoclinostatic test for the examined patients. To assess the vegetative parameters of the nervous system, we also determined the Kerdo vegetative index (VI) for the examined patients [2].
3. Results and Discussion
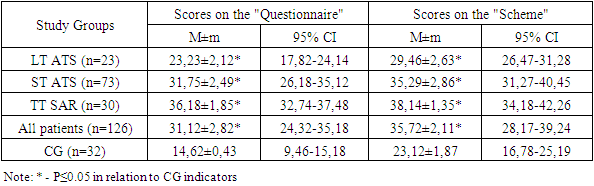
According to the VAS scores, patients according to the severity of the course of exacerbation of SAD were divided into mild course (LT) - 23 (18.25%), moderate severity (SM) of the course of SAD - 73 (57.94%) patients and severe course (TT) of SAD - 30 (23.81%).In patients with LT SAD, the average duration of exacerbation was 24.78±3.42 days, with TS SAD - 31.89±3.87 days, and with TT SAD - 46.88±4.12 days. At the same time, the number of exacerbations of SAR in patients with LT averaged 1.23±0.48 times per year, in the group with STS for SAD - 1.65±0.72 times per year, with TT SAD - 2.34±0 .95 times a year.According to the results of studies of the general detailed analysis of venous blood of patients with exacerbation of SAD, in 85.71% (108 people) we stated an increase in the number of eosinophils within 9-12%, in the remaining 14.29% (18 patients) within 6-8%.In the study of the level of total IgE in the blood serum in 79 (62.70%) patients with SAD, its increase was revealed. There were no statistically significant differences in IgE levels depending on the place of residence. With an increase in the severity of the course of SAR, the concentration of total IgE also increases (P≤0.05). The median level of total IgE in patients with RT SAD was 117.4 (52.69; 195.63) IU/ml, in the ST group for SAD it was 197.12 (82.63; 478.53) IU/ml, in the TT group ATS - 301.29 (127.42; 678.56) IU/ml), while in the CG - 46.37±5.63 IU/ml.The average score of the patients with SAD observed by us according to the "Questionnaire" was 31.12±2.82 (95% CI = 24.32-35.18), and the average score according to the "Scheme" was 35.72±2.11 (95 %CI=28.17-39.24) (P≤0.05) (Table 1).Table 1. IWT status of the studied SAD patients
 |
| |
|
Based on the data obtained, we concluded that there was a statistically significant difference in the IWT indicators of the studied patients with SAD relative to the CG indicators according to the "Questionnaire" and "Scheme", and the indicators of all SAD severity groups were statistically significantly different (P≤0.05).Evaluation of IWT of practically healthy CG individuals by this technique allowed us to determine that normotonia is characterized by up to 6 (5.13±0.18) parasympathotonic and up to 2 (1.28±0.98) sympathotonic signs.У исследуемых пациентов с САР по пробам Даньини-Ашнера норм normal heart rate - in 8 (6.35%) patients, deceleration - in 107 (84.92%), including 1-3 beats - in 11 (8.73%), 4-10 beats - in 25 (19.84%), more than 10 strokes - in 71 (56.35%) of the examined. An increase in heart rate was noted in 11 (8.73%) patients.In the majority of patients (84.92%), a deviation from the normal response was noted in the Dagnini-Ashner test: reduced autonomic reactivity was noted in 25 (19.84%) patients, increased - in 71 (56.35%), perverted - in 11 (8.73%).The average value of heart rate deceleration in the patients we observed was 11.2±0.6 in general, which significantly (p≤0.05) differs from the data obtained during the examination of the CG (4.70±0.68). The slowing of heart rate in the Dagnini-Ashner test according to the Galyu formula averaged 14.5±0.8 beats.Insufficient VOD was recorded in 14 (11.11%) patients with SAD, more often in patients, mainly with a duration of pathology of up to 3 years. Excessive VOD was detected in 80 (63.49%) patients and manifested itself as a hyperdiastolic variant, mainly with a SAR duration of more than 3 years.The studied patients with SAD had the following variants of IWT: normotonia in 22.22% of cases (28 patients), parasympathotonia in 57.14% (72 patients) and sympathotonia in 20.63% of cases (26 patients) (Figure 1).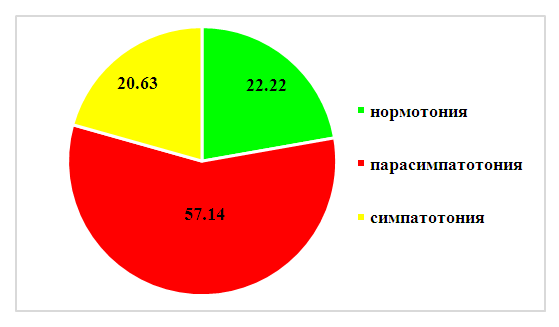 | Figure 1. Variants of IWT of the studied patients with SAD, % |
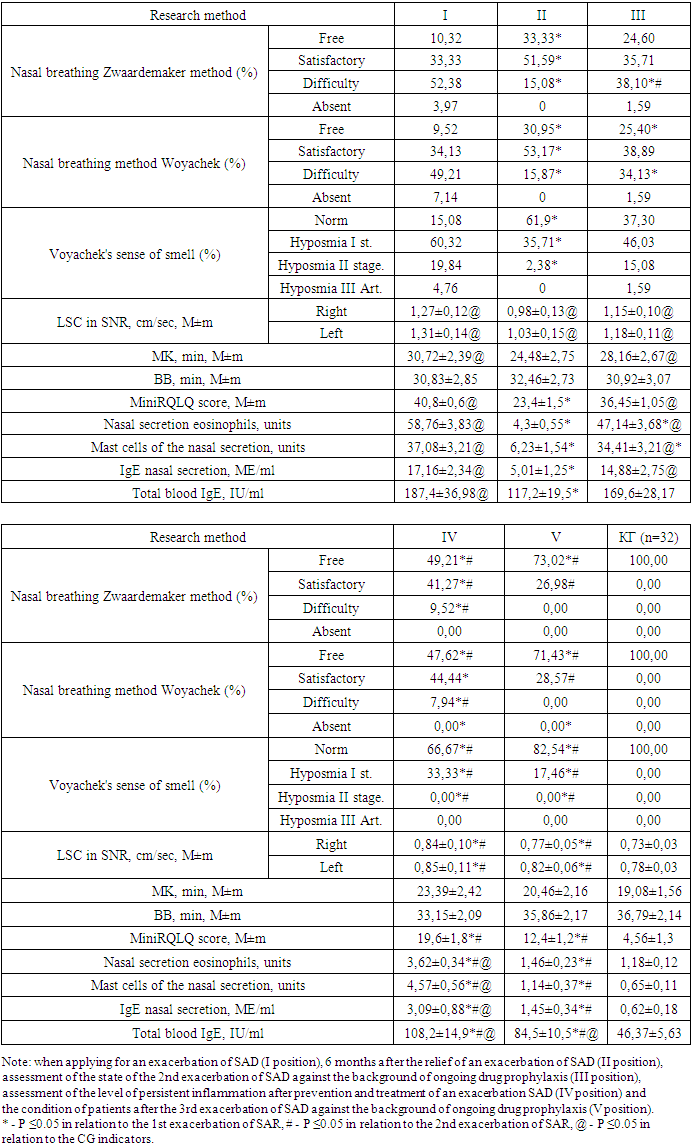
In 72 (57.14%) patients with SAD, the number of vagotonic signs averaged 9.6±1.13 with a constant of sympathotonic signs (1.19±0.12), their IVT was vagotonic.In 26 (20.63%) subjects with SAD, an average of 5.19 ± 1.03 sympathicotonic signs was noted, which is higher than the indicator of practically healthy individuals in the CG and patients with SAD with normotension, while they had an average of 4.83 ± 1, 08 vagotonic signs - their IWT was sympathotonic.Based on the results of the ANS study, it should be concluded that in patients with exacerbation of SAD, shifts in autonomic homeokinesis are noted, characterized in most patients (57.14%) by initial vagotonia in combination with predominantly excessive provision of hyperdiastolic activity. It should be especially noted that with vagotonia, a more severe course of SAR was noted.In our study, for the treatment and prevention of severe exacerbations of SAD, the generally recognized stepwise AR therapy according to ARIA was used [11,19]. In patients with TS and TT SAD, upon obtaining the effectiveness of "clinical recovery", they carried out a "descent one step down" and continued treatment for another 2 weeks until complete remission of the SAD exacerbation.During SAD exacerbation, in order to prevent exposure to allergens in and quickly remove them from the nasal mucosa, we performed elimination-irrigation therapy with a nasal shower of 0.9% sodium chloride solution in all patients with SAD exacerbation (100-200 ml from a comfortable temperature for 1 session ) 2-3 times a day. Some patients, on our recommendation, used the Breather AirOx apparatus of the VP-M3 series for elimination-irrigation therapy, which creates 5 μm aerosol particles from a 0.9% sodium chloride solution (4 ml in a Breather Barrier plastic ampoule) to eliminate allergens and moisturize mucous membrane of the nasal cavity, due to its compactness and convenience, we could recommend patients to use it throughout the day hourly, and most of the patients adhered to compliance. Patients used the Breather AirOx apparatus throughout the day, and at home they also used a nasal shower if desired.Taking into account the peculiarities of the predominance of autonomic innervation in the studied patients with SAD, we applied the correction of the predominance of the ANS, in particular in vagotonics (57.14% (72 patients)), correction of eutonia (22.22% of cases (28 patients)) and sympathotonia (20.63% cases (26 patients)) were not performed.The effectiveness of our drug therapy for SAD patients was assessed by us according to the totality of clinical symptoms as "clinical recovery", "clinical improvement", "no effect", "clinical deterioration".It should be taken into account that all 30 patients with TT SAD were vagotonic and 42 out of 73 patients (57.53%) with TS of SAD were also vagotonic. In 72 patients with vagotonic IWT, we used a modified scheme of stepwise pharmacotherapy of AR from ARIA.Stage II of therapy was applied by us in all patients with vagotonia, while the effect was achieved by us in 51 patients (70.83%) - in all vagotonic patients with STS of the course of SAD and 7 patients with vagotonics with TT SAD. In 21 (29.17%) patients with TT SAR, we used stage III AR therapy, which caused clinical improvement in all patients.As part of the complex therapy of SAD in patients with vagotonia, we carried out therapeutic measures to reduce the parasympathetic prevalence of the ANS and stimulate sympathetic innervation: Grandaxin (Tofisopam) 50 mg orally, 1 tablet (50 mg) 30 minutes before meals 2 times a day; A solution of calcium chloride 10% diluted 1:1 with a 0.5% solution of novocaine - endonasal electrophoresis for 5 minutes and segmental ionogalvanization according to A.E. Shcherbak for 10 minutes lasting 5-10 days.The course of vegetotropic treatment (medication and physiotherapy) was repeated every 6 months without SAD exacerbations and without fail 2 weeks before the next SAD exacerbation in parallel with prophylactic treatment of AR (stage II of the ARIA stepwise therapy) starting from the second exacerbation.Thus, throughout the study period, all the studied patients received 1 course of SAD treatment at the time of admission, after which their condition was assessed and corrections were made in subsequent courses and 2 courses of SAD treatment against the background of prophylactic treatment of SAD exacerbation, vagotonics received corrective ANS therapy and beyond. exacerbations. Therapeutic measures were prescribed, strictly adhering to the indications and stages of ARIA, based on the severity of the manifestation of AR in each specific exacerbation of SAD.Study patients with vagotonia received 5 courses of vegetotropic treatment (1st study exacerbation, 6 months after it, 2nd study exacerbation and 6 months after it, study 3 exacerbation).Thus, in our study, several follow-up positions were formed - when applying for an exacerbation of SAD (I position), 6 months after the relief of an exacerbation - conducting scarification tests and assessing the level of persistent inflammation after treatment of an exacerbation of SAD (II position), assessing the state of the 2nd exacerbation SAD against the background of ongoing drug prophylaxis (III position), assessment of the level of persistent inflammation after the prevention and treatment of exacerbation of SAD (IV position) and the condition of patients after the 3rd exacerbation of SAD against the background of ongoing drug prophylaxis (V position).In follow-up position I, we assessed the condition of patients with SAD without prophylactic treatment before exacerbation and correction of the state of the ANS. During the time from the exacerbation of SAD at the time of admission of patients to the assessment of their condition, all patients underwent scarification tests and studies of the state of the ANS. Thus, based on the data obtained, we could assess the reasons for the unsatisfactory effectiveness of the treatment of SAD exacerbation.All the functional and laboratory parameters studied by us decreased outside the exacerbation of SAD, however, almost none of the parameters reached the CG level, which unequivocally confirms the presence of persistent allergic inflammation in our patients.This circumstance prompted us to pay attention to the state of the ANS, after which we prescribed 72 (57.14%) patients with a predominance of parasympathotonia to correct this state of therapy.Based on the obtained statistically significant differences in the results of SAD preventive therapy and corrective ANS therapy in patients with vagotonia predominance, we came to an unambiguous conclusion about an increase in the effectiveness of complex treatment.We unequivocally stated an improvement in nasal breathing in patients with SAD after the use of complex therapy, so such indicators as nasal breathing according to the Zwaardemaker and Woyachek method changed statistically more effectively relative to the first exacerbation - the number of patients with free and satisfactory breathing increased, there were no patients with poor nasal breathing and its absence, the number of patients with a normal sense of smell also significantly increased - 82.54% in position V relative to 61.9% in position II.Table 2. Comparison of the state of patients with SAD in exacerbation and beyond it by functional and laboratory methods
 |
| |
|
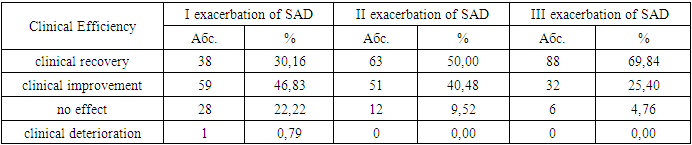
In the group of patients with vagotonia and SAD, a significant (p<0.05) moderate direct correlation was found between the strength of the predominance of parasympathotonia in the second position of catamnesis and rhinorrhea (r=0.53), eyelid edema (r=0.54), nasal congestion (r=0.61); significant (p<0.05) moderate inverse correlation between the strength of parasympathotonia predominance and the frequency of good treatment outcomes (r= -0.54).In the group of patients with vagotonia and TT SAR, a significant (p<0.05) moderate direct correlation was recorded between the scores of the miniRQLQ questionnaire in SAD exacerbation with the frequency of unsatisfactory nasal breathing (r=0.41), with the degree of hyposmia (r=0, 38), LSC according to SNR (r=0.37). In the same group of patients with SAD, a significant (p<0.05) average direct correlation was found between the duration of SAD exacerbation and the levels of eosinophils (r=0.48), mast cells (r=0.51) and IgE (r=0 .49) in nasal secretion, as well as scores on the miniRQLQ questionnaire (r=0.42) and serum IgE (r=0.41).Statistical analysis in the group of patients with TS SAD and vagotonia stated a statistically significant (p<0.05) moderate direct correlation between the miniRQLQ scores in SAD exacerbation and the frequency of rhinorrhea (r=0.55), nasal congestion (r=0 .58), itching in the eyes (r=0.57), itching in the nasal cavity (r=0.56) and pollen intoxication (r=0.53). A moderately strong direct correlation was also recorded between UA and eosinophils (r=0.54), mast cells (r=0.52) and IgE (r=0.51) in the nasal secretion; Significant (p<0.05) average inverse correlation between VV and miniRQLQ scores (r= -0.50). A statistically significant (p<0.05) average direct correlation between the degree of nasal breathing and scores on the miniRQLQ questionnaire (r=0.60) was also revealed; significant (p<0.05) moderate inverse correlation between VV in exacerbation and the incidence of eyelid edema (r= -0.52), nasal congestion (r= -0.53), rhinorrhea (r= -0.51).According to the results of the study, we stated that the worst results of the first treatment were found in groups with TT and CT of the course of SAD, which is quite expected and predictable. However, it should be noted that all patients from the TT group (n=30) were characterized by a predominance of parasympathotonia, an identical state of the prevalence of ANS was also characterized by 42 out of 73 patients of the TS SAR group, which accounted for 57.53% of this group, and 31 patients of the TS group and all 23 patients with LT SAD were characterized by eutonia and sympathotonia.It should be noted that it was the patients with vagotonia who demonstrated the worst initial efficacy of the therapy in the first position of follow-up, i.e. after initial treatment and treatment. Patients with TT and CT of the course of SAD and during the period of remission were characterized by large impairments in QoL, which, in our opinion, is a strong evidence of a higher degree of persistent allergic inflammation in such patients compared to LT SAD, which is also explained by the predominance of the vagotonic type of IWT in them.Carrying out corrective vagotonia therapy allowed us to further significantly increase the effectiveness of antiallergic stepwise treatment in these patients, which undoubtedly proves a significant effect of parasympathetic IWT both on the level of persistent allergic inflammation and on the severity of seasonal exacerbations of the disease.Analyzing the results of the complex therapy of three SAD exacerbations according to the characteristics of efficiency gradations, the studied patients were distributed differently after each SAD exacerbation.Table 3. Evaluation of the clinical efficacy of complex therapy for exacerbations of SAD
 |
| |
|
Thus, after 1 exacerbation, we achieved a positive effect in 97 (76.98%) patients, with complete relief of clinical symptoms only in 38 (30.16%) patients, i.e. 39.18% of them. The corrective predominance of therapy as part of complex treatment allowed us to achieve better results already after the next exacerbation of SAD - a positive effect was already noted in 114 (90.48%), and we achieved complete relief of the clinical picture in 63 patients - 55.26% of them, and repeated courses of correction of IWT led to the effectiveness of the treatment of exacerbation of SAD in 120 (95.24%), against the background of complete relief of the clinic in 88 patients - 73.33% of them.
4. Conclusions
1. According to the severity of the exacerbation of seasonal allergic rhinitis, 18.25% had a mild course, 57.94% had an average severity of the course, and 23.81% had a severe course of the disease.2. In 57.14% of patients with seasonal allergic rhinitis, initial vagotonia was found in combination with predominantly excessive provision of hyperdiastolic activity.3. The use of corrective vegetotropic therapy allows to increase the effectiveness of the treatment of exacerbation of SAD in vagotonics (57.14% of all patients with SAD) by 1.21 times in relation to the total clinical effectiveness and by 1.64 times in relation to the clinical recovery of patients with SAD. The average increase in the effectiveness of the complex treatment of SAD, including the correction of vagotonia, is 1.42 times.
References
| [1] | Abdurakhmanova A.A., Belozerov Yu.M., Makkaev Kh.M. Assessment of the vegetative status and hemodynamic parameters in vasomotor and allergic rhinitis in children // Russian otorhinolaryngology - Moscow, 2006. - No. 4 (23). –S.39-44. |
| [2] | Wayne A.M. Vegetative disorders: clinic, diagnosis, treatment. - M.: Medical Information Agency, 2003. - 377 p. |
| [3] | Volkov A.G. et al. The role of autonomic dysfunction in the pathogenesis of diseases of the ENT organs // Russian otorhinolaryngology - Moscow, 2004. - No. 3 (10). – P.15–18. |
| [4] | Daliev A.G. Clinical and functional aspects and improvement of the treatment of allergic rhinitis in children of school age: Diss…dokt. honey. Sciences., Tashkent 2020, 193s. |
| [5] | Danilov A.B. Peripheral autonomic failure // Journal of Neurology and Psychiatry - Moscow, 1997. - Volume 97 No. 2. - P. 44-50. |
| [6] | Zaichik A.Sh., Churilov L.P. Fundamentals of general pathology. Part 1. Fundamentals of General Pathophysiology. Textbook for medical universities.: St. Petersburg: Elbi, 1999. - 624 p. |
| [7] | Naumenko N.N., Shustova T.I., Konoplev O.I. Dysfunction of the autonomic nervous system in patients with diseases of the upper respiratory tract // XVII Congress of Otorhinolaryngologists of Russia: Materials of the Congress - St. Petersburg, 2006 - P. 310-314. |
| [8] | Nenasheva N.M., Shilenkova V.V. Control of symptoms of allergic rhinitis in adults in the Russian Federation: results of an online survey // BC. Medical review. - 2021. - V. 5, No. 1. S. 25-31. |
| [9] | Smirnov D.S., Kurbacheva O.M. A modern view on the treatment of allergic rhinitis in combination with bronchial asthma. // Medical advice. 2021. - No. 6. - p.92-98. |
| [10] | Stagnieva I.V. Autonomic dysfunction in the manifestation of prosopalgia in patients with rhinosinusitis. // Medical Bulletin of the South of Russia - Rostov-on-Don, 2012. - No. 2. - P. 67 - 69. |
| [11] | Brożek JL, Bousquet J, Agache I, Agarwal A. et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines-2016 revision. // J Allergy Clin Immunol. 2017. - No. 140 (4). - r.950-958. doi: 10.1016/j.jaci.2017.03.050. Epub 2017 Jun 8. PMID: 28602936. |
| [12] | Doulaptsi M, Wils T, Hellings PW, Martens K, et al. Mometasone furoate and fluticasone furoate are equally effective in restoring nasal epithelial barrier dysfunction in allergic rhinitis. // World Allergy Organ J. 2021 Sep 11. - №14(9). – р.100585. |
| [13] | Epstein T.G., Murphy-Berendts K., Liss G.M. et al. Risk factors for fatal and nonfatal reactions to immunotherapy (2008-2018): post-injection monitoring and severe asthma // Ann Allergy Asthma Immunol. - 2021. - Mar 19: S1081-1206(21) 00187-3. |
| [14] | Gianni M. Evolution Of Immunotherapy Against Pollen Allergy. Curr Protein Pept Sci. 2023 Mar 3. doi: 10.2174/1389203724666230303091754. |
| [15] | Ihua M. Decaying Ascophyllumnodosum as a source of algal cell wall degrading enzymes with potential utility in enzyme-assisted extraction technologies // Access Microbiology. – 2019. – Vol. 1, № 1A. – P. 555–557. |
| [16] | Lal D., Corey J.P. Vasomotor rhinitis update // Curr. Opin. Otolaryngol. Head Neck Surg. – 2004. – Vol. 12, № 3. – P. 243-247 |
| [17] | León B., Ballesteros-Tato A. Modulating Th2 Cell Immunity for the Treatment of Asthma // Front Immunol. - 2021. - Vol. 12: 637948. |
| [18] | Mulkey SB, Plessis AJ. Autonomic nervous system development and its impact on neuropsychiatric outcome. // Pediatr Res. 2019. - № 85 (2). – с. 120–126. |
| [19] | Onerci Celebi O, Araz Server E, Kirgezen T, Yigit O, Aki ES. Intranasal Schirmer Test in Allergic Rhinitis: Relationship to Symptom Scores and Role in Determining Response to Treatment. // Ann Otol Rhinol Laryngol. 2023. - №4. – р. 3489-3497. doi: 10.1177/00034894231176327. Epub ahead of print. PMID: 37271974. |
| [20] | Van Gerven L., Steelant B., Hellings P.W. Nasal hyperreactivity in rhinitis: A diagnostic and therapeutic challenge. // Allergy. – 2018. - №73(9). – р.1784-1791. |
| [21] | Weaver-Agostoni J, Kosak Z, Bartlett S. Allergic Rhinitis: Rapid Evidence Review. // Am Fam Physician. 2023 May. - №107(5). – р.466-473. PMID: 37192071. |
| [22] | Wiernsperger N., Rapin J. R. Microvascular Diseases: Is A New Era Coming? Cardiovasc Hematol Agents // Med Chem. 2012. - №6. – с.167–183. |



 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML

