-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2023; 13(8): 1143-1149
doi:10.5923/j.ajmms.20231308.23
Received: Aug. 8, 2023; Accepted: Aug. 26, 2023; Published: Aug. 28, 2023

Influence of Hyperprolactinemia on Immunohistochemical and Morphological Characteristics of Breast Cancer
Khalimova Z. Yu., Gumarova A. A.
Republican Specialized Scientific and Practical Medical Center of Endocrinology Named after Academician Y. Kh. Turakulov, Tashkent, Uzbekistan
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The review discusses the data from contemporary literature and evaluates the contribution of hyperprolactinemia to the development of breast cancer. The correlation between a high frequency of adverse histological subtypes, such as invasive ductal carcinoma, adenocarcinoma, medullary cancer, and others, with hyperprolactinemia has been identified. Additionally, a relationship has been established between relatively unfavorable molecular subtypes of breast cancer and hyperprolactinemia (triple-negative, non-luminal HER2/neu).
Keywords: Hyperprolactinemia, Breast cancer, Immunohistochemistry, Pituitary adenoma, Prolactinoma, Histological subtypes of breast cancer, Molecular subtypes of breast cancer
Cite this paper: Khalimova Z. Yu., Gumarova A. A., Influence of Hyperprolactinemia on Immunohistochemical and Morphological Characteristics of Breast Cancer, American Journal of Medicine and Medical Sciences, Vol. 13 No. 8, 2023, pp. 1143-1149. doi: 10.5923/j.ajmms.20231308.23.
Article Outline
1. Introduction
- Hyperprolactinemia is one of the most common endocrine disorders and can affect both genders at any age. The annual incidence of hyperprolactinemia in women is 8.7 per 100,000 individuals [5], and in the age group of 25-34 years, it increases to 29.3 cases per 100,000 population [3]. The most common causes of hyperprolactinemia include pituitary adenoma, intracranial tumors, medication use, primary hypothyroidism, and chronic kidney failure. Hyperprolactinemia is responsible for menstrual dysfunction in 10% of cases and infertility in 25-40% of cases [4]. According to the World Health Organization (WHO), malignant neoplasms represent the largest burden for women worldwide, with 19.6 million DALYs (Disability-Adjusted Life Years) attributed to breast cancer. Breast cancer is the most frequently diagnosed malignant neoplasm in women globally, with 2.26 million new cases registered in 2020 [95% CI, 2.24–2.79 million] [2]. The prevalence of breast cancer in the context of hyperprolactinemia has not been fully studied. However, the impact of prolactin on mammary glands changes is still not fully understood, as research by various authors has yielded conflicting results. A search on the National Medical Library website using keywords such as prolactin, breast cancer, breast disease, galactorrhea, breast revealed 7433 references [1]. Nevertheless, the most extensively studied effects of prolactin remain its influence on the reproductive organs, especially the mammary gland. Studies related to prolactin have far exceeded those investigating any other hormone in the blood plasma of women with breast cancer or at high risk of developing the disease. Early studies aimed to identify connections between elevated prolactin levels and breast conditions, particularly the association between breast cancer and prolactin levels. There is substantial indirect and direct evidence of prolactin's involvement in carcinogenesis. Most researchers note its association with the severity and duration of the disease [2,5]. Overall, epidemiological studies indicate a significant correlation between high circulating prolactin levels and the risk of developing breast cancer.
2. Purpose of the Study
- To study the relationship of hyperprolactinemia with the immunohistochemical and morphological characteristics of breast cancer in women, and determine the tactics of their management.
3. Materials and Methods
- The study included 100 female patients with breast cancer associated with hyperprolactinemia. To achieve the set objective, all patients were divided into two groups: Group I - 70 women with hyperprolactinemia associated with breast cancer, and Group II - 30 patients with breast cancer and normal prolactin levels. All patients underwent clinical examinations, history-taking (with a focus on the age at menarche, cycle regularity, use of oral contraceptives, parity, history of childbirth and pregnancies, breastfeeding, age and duration of menopause), physical examination, and clinical manifestations. The palpation of the breasts was performed to assess lactation and the presence of masses, as well as changes in nipple and areolar condition, thickening or density, and the presence or absence of nipple discharge and its characteristics. Hormonal status was evaluated by measuring prolactin, estradiol (E2), luteinizing hormone (LH), follicle-stimulating hormone (FSH), progesterone, testosterone, thyrotropin, and free thyroxine levels using immunochemiluminescent assays (IHCL). Histological status was determined for all patients through core biopsies, aspiration and incisional biopsies of the breast, and immunohistochemical (IHC) examination, with the expression of ER, PR, HER-2, and Ki-67 receptors being determined.Imaging methods included ultrasound examination of the breasts, mammography, and whole-body magnetic resonance imaging (MRI) to detect pituitary adenomas, neoplasms, and the presence of metastases. The inclusion criteria for the study were as follows: women with elevated prolactin levels above 23.3 ng/mL in at least two measurements, confirmed breast cancer, age of the patient between 19 and 65 years. The exclusion criteria included the presence of concomitant endocrine disorders such as hypothyroidism, thyrotoxicosis, type 1 and 2 diabetes, cessation of breastfeeding at least 2 years before the study, stage III breast cancer, liver cirrhosis, and medication use (dopamine agonists, psychotropic drugs, etc.) within a month before the study. For the statistical analysis of the study results, data from all 100 patients were entered into a "database" created using electronic Excel spreadsheets. The obtained results were processed using standard IBM SPSS Statistics packages.
4. Results
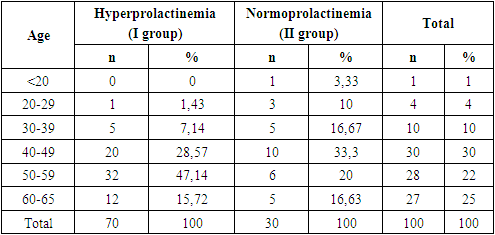
- The study included 70 patients with hyperprolactinemia associated with breast cancer (Group I) and 30 women with breast cancer without hyperprolactinemia (Group II, the control group). The age of patients in the first group ranged from 29 to 65 years (mean age in the main group was 49 ± 11.7). In Group II, the age ranged from 19 to 65 years (mean age was 45.2 ± 13.2). Table 1 presents the age distribution of breast cancer patients based on prolactin levels.
|
 | Figure 1. Brain changes in MRI examination of Group I |
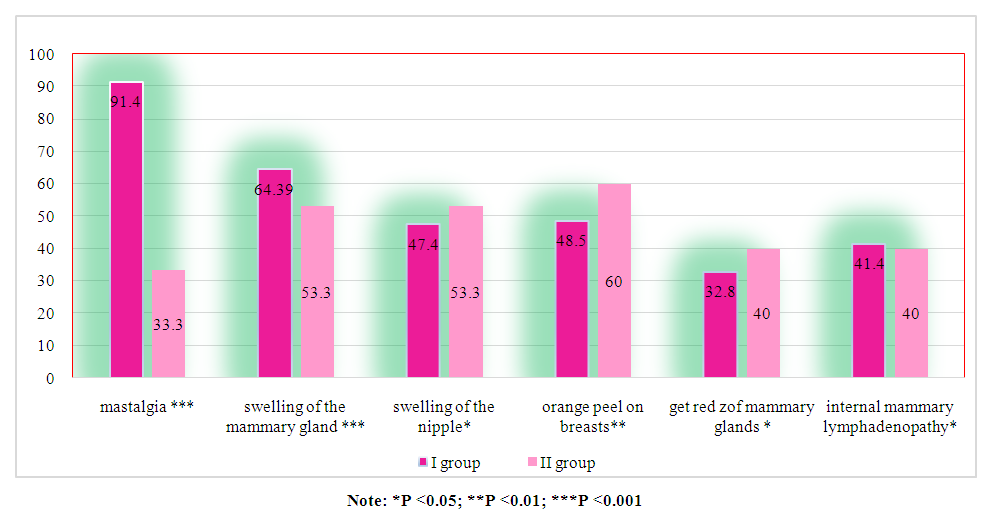 | Figure 2. Frequency of occurrence of main symptoms in patients of Group I and II in comparative aspect (n=100) |
|
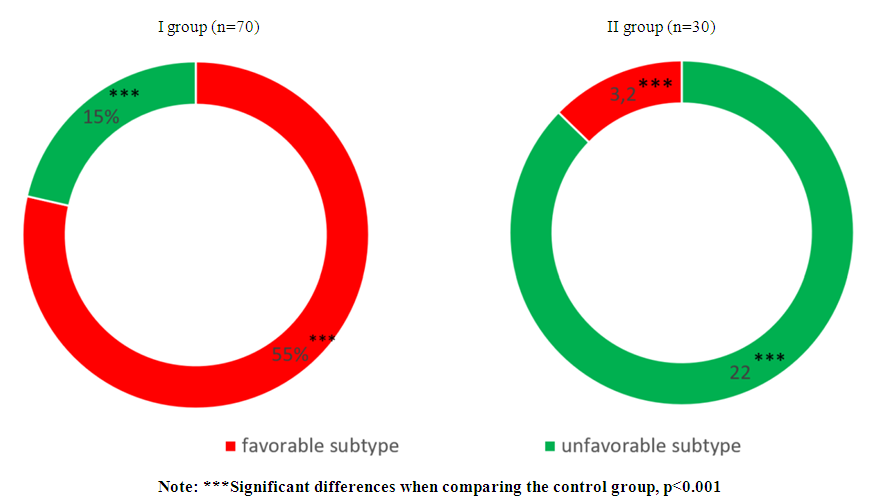 | Figure 3. Distribution of breast cancer histological subtypes depending on the level of prolactin |
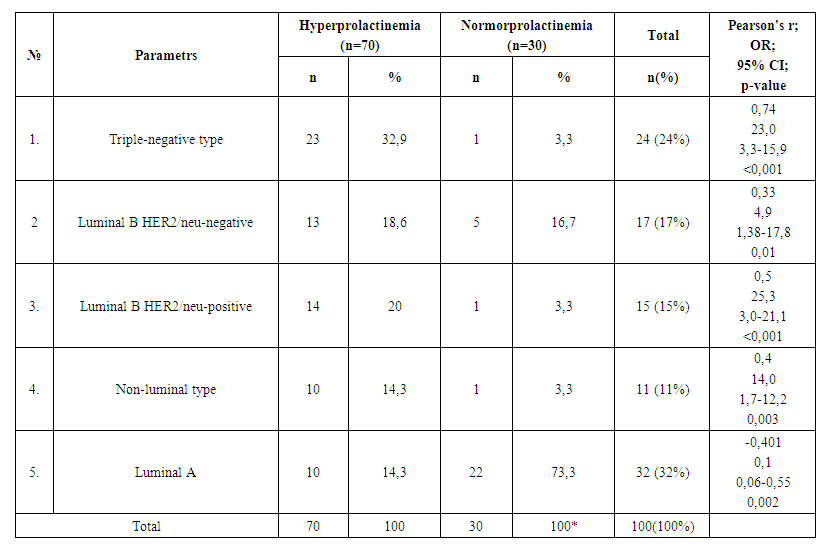 | Table 3. Molecular Subtypes of Breast Cancer in Normal and Hyperprolactinemia Conditions |
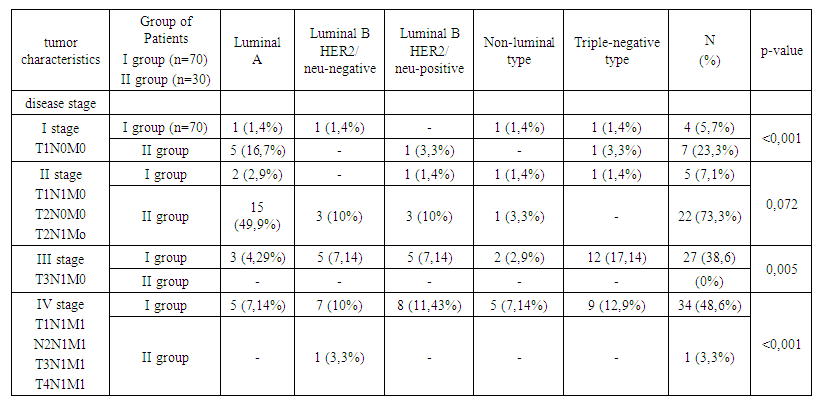 | Table 4. Frequency of occurrence of molecular subtypes depending on the stage of breast cancer and prolactin level |
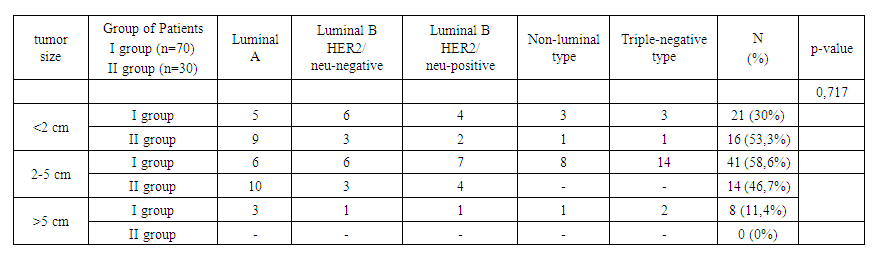 | Table 5. Study of molecular subtypes and tumor size in breast cancer depending on prolactin level |
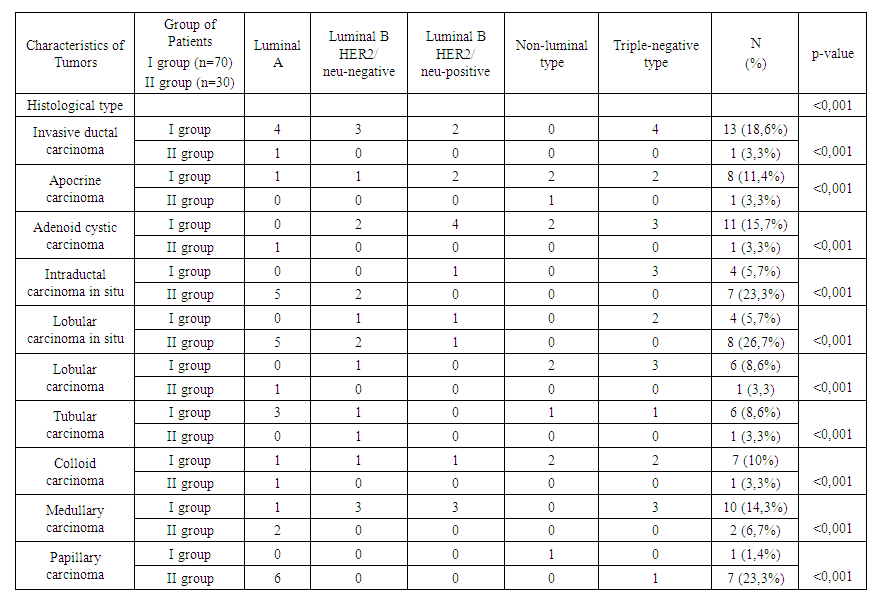 | Table 6. Frequency of breast cancer occurrence considering molecular and histological subtypes depending on prolactin level (n=100) |
5. Conclusions
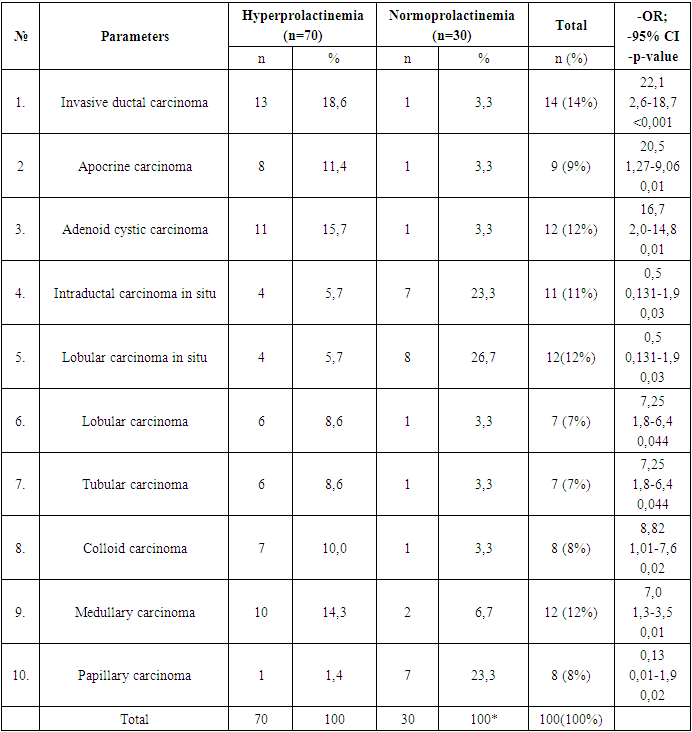
- Analysis of clinical and laboratory indicators in women with hyperprolactinemia (Group I) associated with breast cancer (BC) revealed a significant predominance of mastalgia (91.4%), nipple edema (53.3%), and breast edema (53.3%) accompanied by the lactoreia-dysmenorrhea syndrome. In contrast, Group II showed a significant prevalence of classical breast cancer symptoms, such as bloody nipple discharge (92%), orange-peel skin (60%), breast redness (40%), and axillary lymph node enlargement (40%). The study of the correlation between histological and molecular subtypes with prolactin levels revealed a predominance of invasive ductal carcinoma in 13 cases (19%), adenocarcinoma in 11 cases (16%), and medullary carcinoma in 10 cases (14.3%) in Group I. In contrast, Group II showed an increased frequency of in situ intraductal and papillary carcinoma in 7 cases (23.3%) and 8 cases (26.7%) respectively, compared to 5.7%, 5.7%, and 1.4% in hyperprolactinemia (p<0.001). Comparative study of the immunohistochemical characteristics of the receptor status in breast cancer tissue in the investigated groups revealed a significant increase in HER2/neu (67.2%) and Ki-67 (52.7%) in Group I compared to (9.9%) and (9.6%) respectively in Group II (p<0.001). Estrogen receptors (ER) and progesterone receptors (PR) predominated significantly in women of Group II, with 81.6% and 59.6%, respectively, compared to 24.8% and 19.3% in Group I (p<0.001). These findings suggest that in cases of normal prolactin levels, breast cancer is highly differentiated with low proliferative activity and minimal aggressive progression. The study of the immunohistochemical characteristics of the receptor status of breast tissue, taking into account the prolactin level and molecular subtype of the tumor, revealed a predominance of triple-negative subtype in 32.9% compared to 3.3%, as well as non-luminal subtype in 20% compared to 3.3% in Group I. Conversely, in Group II, the majority of cases were luminal A in 73.3% compared to 14.3% of molecular subtypes (p<0.001). The correlation analysis between prolactin levels and clinical, hormonal, histological, molecular subtypes, and immunohistochemical indicators showed a positive correlation with mastalgia (r=0.71), Ki-67 (r=0.6), HER2/neu (r=0.7), metastasis (r=0.56), estradiol (0.63), and invasive ductal carcinoma (r=0.6) and adenocarcinoma (r=0.5), while showing a negative correlation with ER (-0.67) and PR (-0.59), as well as papillary carcinoma (r=-0.78), in situ ductal carcinoma (r=-0.69), and in situ intraductal carcinoma (r=-0.65) respectively, with a significance of p=0.001.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML