-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2023; 13(8): 1118-1122
doi:10.5923/j.ajmms.20231308.18
Received: Aug. 3, 2023; Accepted: Aug. 21, 2023; Published: Aug. 23, 2023

The Effect of Body Mass Index on the Clinical and Morphological Characteristics of Breast Cancer in Women with Metabolic Syndrome
L. T. Alimkhodjayeva1, M. A. Mirzayeva2, L. T. Zakirova1, M. X. Norbekova2
1Republican Specialized Scientific and Practical Medical Center of Oncology and Radiology
2Tashkent Medical Academy, Tashkent, Uzbekistan
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The aim of the study was to determine the effect of body mass index and menopausal statuses on the development of breast cancer and its molecular subtypes. Background. Obesity is not only one of the risk factors leading to breast cancer, but how obesity accompanies cancer is crucial for evaluating the effects of the disease. This article examines the role of obesity in the occurrence and course of breast cancer in women, as well as its dependence on the molecular subtypes of the tumor and the state of menopause in women; inflammatory cytokines and their involvement in the mechanisms of tumor development and progression are considered. Material and methods. The retrospective study was conducted from February 2018 to August 2022 in Tashkent, at the Republican Specialized Scientific and Practical Medical Center of Oncology and Radiology. We selected women with obesity of the II-IV clinical +++ stages of breast cancer who applied to the central polyclinic of the Republican Specialized Scientific and Practical Center of Oncology and Radiology. The patients were divided into two groups depending on the menopausal status of women: the first group included 43 postmenopausal women, the second group included 42 premenopausal women. Results. Her2/neu positive breast cancer occurred in the premenopausal group more often than other molecular subtypes (45%). In the postmenopausal group, the triple negative subtype (35.5%) and her2/neu positive subtype (32.25%) were detected more frequently than the other histological subtypes (luminal). In obese patients, the size of the tumor decreases more slowly than in patients with normal and overweight. This condition is especially noticeable in the postmenopausal group. Conclusion. It was found that obesity was associated with an increased risk of her2/neu tumors and correlated with low median metastasis-free survival among premenopausal patients and with triple negative cancer among postmenopausal patients. A decrease of the body mass index in patients with breast cancer leads to an improvement of the disease outcomes and a decrease in the resistance of the tumor to chemotherapy.
Keywords: Breast cancer, Body mass index, Premenopausal, Postmenopausal, Triple negative, Estrogen receptor, Progesterone receptor
Cite this paper: L. T. Alimkhodjayeva, M. A. Mirzayeva, L. T. Zakirova, M. X. Norbekova, The Effect of Body Mass Index on the Clinical and Morphological Characteristics of Breast Cancer in Women with Metabolic Syndrome, American Journal of Medicine and Medical Sciences, Vol. 13 No. 8, 2023, pp. 1118-1122. doi: 10.5923/j.ajmms.20231308.18.
1. Introduction
- Obesity is a serious public health problem, mainly in developed countries. This is associated with cancer risk and mortality. According to the WHO classification, there are three degrees of obesity. Body mass index (BMI) 30-34.9 kg/m2 - I degree, 35-39.9 kg/m2 - II degree and ≥40 kg/m2 - III degree of obesity. In obesity, adipose tissue produces free fatty acids and various hormones of both metabolic and endocrine organs, such as leptin, adiponectin, adipokin, tumor necrosis factor α (TNF-α) and others, which are involved in the oncogenic process.It has been found that body mass index (BMI) is associated not only with the incidence but also with the prognosis of breast cancer. As an increase of BMI is positively associated with the risk of breast cancer in postmenopausal patients, but not in premenopausal patients [1-3], such an increase seems to be associated with the incidence of breast cancer, possibly due to an increase in the production of circulating estrogens [4]. This hypothesis appears to be supported by reports of a positive association between BMI and the risk of ER-positive breast cancer in postmenopausal women [5-6]. It was further reported that increased BMI is largely associated with a worse prognosis, especially for patients in the pre-/perimenopausal period [2]. According to this finding, it was revealed that a higher BMI was associated with an increase in mortality in premenopausal patients. Their analysis showed a positive association between a higher BMI and a worse prognosis for patients with hormone receptor-positive (er+) breast cancer [2,7]. Besides, the increased BMI in their study correlated with a worse prognosis only for ER-positive and for human epidermal growth factor receptor 2 (HER2)-negative, but not HER2-positive or thrice-negative tumors, regardless of menopausal status [6,8,9]. The aim of the study was to determine the effect of body mass index and menopausal statuses on the development of breast cancer and its molecular subtypes.
2. Material and Methods
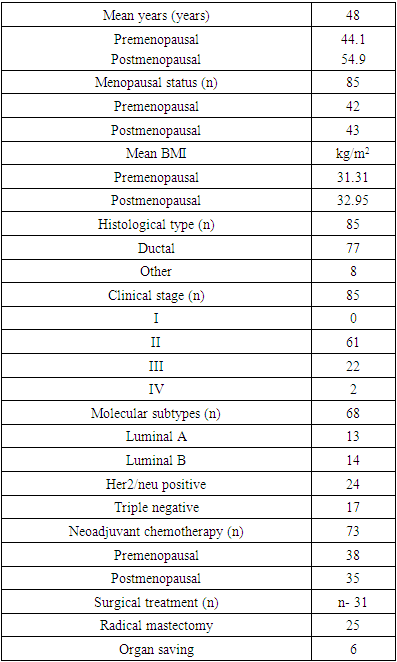
- The retrospective study was conducted from February 2018 to August 2022 in Tashkent, at the Republican Specialized Scientific and Practical Medical Center of Oncology and Radiology (RSSPMCOR). We selected women with obesity of the II-IV clinical +++ stages of breast cancer who applied to the central polyclinic of the RSSPMCOR. The body mass index of each woman was evaluated in accordance with WHO criteria. The total number of patients included in the study was 85. The patients were divided into two groups depending on the menopausal status of women: the first group included 43 postmenopausal women, the second - 42 premenopausal women. The mean age of all patients made up 48 years; the mean age was 44.1 years and 54.9 years in premenopausal and postmenopausal women, respectively. The youngest patient was 29 years old, the oldest was 69 years old, the average was 49 years.According to BMI, the patients were divided into three subgroups: the first subgroup (n=15) with a BMI of ≤24.9 kg/m2 (women with normal weight; control group); the second subgroup (n=18) of women with a BMI of 25-29.9 kg/m2 (overweight women) and the third subgroup (n=52) women with BMI ≥30 kg/m2 and obesity. Breast cancer tissue was taken from each woman for immunohistochemical examination (IHCH) by trephine biopsy. Then the materials were sent to the histological laboratory and examined using paraffin-embedded monoclonal antibodies (Dako, USA). Tumor markers ER, PR, HER/2 and Ki-67 were evaluated. Other clinical features of patients are shown in Table 1.
|
3. Results
- The number of patients with normal body weight, overweight, obesity in premenopausal women was 10 (23, 25%), 11 (25, 6%) and 22 (51, 1%), respectively, and in the postmenopausal group - 6 (14%), 7(16%) and 29(70%) respectively. The BMI of postmenopausal women was higher than that of premenopausal women.The distribution of molecular subtypes in pre- and postmenopausal women by BMI subgroups was studied. According to the results of the analysis in the group of women in postmenopausal status, the majority of patients had the 1st and 2nd degree of obesity.31 patients were performed the immunohistochemical (IHCH) test in the postmenopausal group. Triple negative subtype -11(35.5%) and HER2/neu positive subtype - 10 (32.25%) were more common than other histological subtypes (luminal), especially in obese patients than in overweight and normal weight patients. Luminal B-histological type was the least common in postmenopausal women in compare with other histological types (9%). There was no cross-correlation between the degrees of obesity (1, 2 and 3 degrees) and histological subtypes. The second group consisted of 42 premenopausal women. 37(90%) patients were performed trephine biopsy and IHCH test. Her2/neu-positive breast cancer was detected in the premenopausal group more often than other molecular subtypes -17 (45,94%) cases. Triple negative breast cancer made up - 6 (16.21%). Premenopausal patients had more luminal subtypes A and B in comparison with postmenopausal (5-13.5% and 9-24.32%, respectively). The difference increases when comparing the frequency of luminal types in two groups of obese patients (p<0.01). In the premenopausal group of patients with a BMI of ≥30 kg/m2, ER and PR negative tumors were more detected compared to patients with overweight and normal weight (p<0.05).Overweight postmenopausal women and obese women were more likely to have luminal subtypes B than patients with normal body weight (Fig. 1).
 | Figure 1. BC molecular subtypes occurrence frequencies in post- and premenopausal groups |
4. Discussion
- In obesity, the effect of breast cancer subtypes on the prognosis of the disease has been studied in many researches, depending on the menopausal status of women. Obesity increases the risk of ER + breast cancer in postmenopausal women and reduces the risk of breast cancer in premenopausal women, according to a study conducted by several authors [1,3]. However, these authors did not consider hormone receptor negative or triple negative subtypes in premenopausal women. Subsequent studies examined the risk of triple negative breast cancer in premenopausal women [8,9,10]. Studies conducted in America in 2011 and 2014 showed that 39% of women over 60 are obese [1]. Obesity increases the risk of metabolic syndrome and postmenopausal breast cancer. The increased risk of postmenopausal breast cancer in obesity has been studied in many investigations. Among 1.2 million women aged 50–64 years in the United Kingdom during the 2011–2014 follow-up period, 45,037 women were diagnosed with breast cancer. In this study, the risk of breast cancer in obese postmenopausal women exceeded 30% [11]. The occurrence of obesity in hormone receptor positive postmenopausal women has been studied in many cohort and case-control studies. Studies of hormone receptor-negative or triple-negative breast cancer subtypes in postmenopausal women have shown no association between these obesity subtypes and breast cancer [11,12].According to the results of studies conducted in the United States in 2011 and 2014, obesity was noted in 35% of women aged 20-59 years. About 20% of breast cancer women were under the age of 50 [1]. One of the largest studies conducted in this regard was conducted on more than 2.5 million women, 7930 of them were premenopausal women with breast cancer. Studies show that every 5 kg/m2 increase in BMI reduces the risk of developing cancer by about 8% [10]. However, not all studies have shown that obesity reduces the risk of premenopausal breast cancer [1,3]. Compared with ER-positive obese premenopausal women, hormone receptor-negative and triple-negative subtypes have a significantly higher risk of the disease. According to two meta-analyses of 620 and 1358 women with thrice-negative breast cancer, the risk of thrice-negative breast cancer in premenopausal obese women is 82% and 43%, respectively [9-10]. Besides, another cohort meta-analysis and a case-control meta-analysis showed that obesity reduced the risk of hormone-receptor-positive breast cancer by 20% among premenopausal women, but this study did not correlate with other subtypes [1,7,13,10,14]. In recent years, case-control studies have shown that the risk of developing an infiltrative form of breast cancer is high in women with a high BMI index, regardless of their menopausal status [3].In our study we determined that BMI was an important factor in tumor response to chemotherapy. It was found that obesity was associated with an increased risk of her2/neu tumors and correlates with poor median survival without metastases among premenopausal patients and with triple negative cancer among postmenopausal patients (five-year survival without metastases made up 81% and 90.7% in premenopausal and postmenopausal groups, respectively). In the postmenopausal group, lung metastases were found in 1 (2.3%) case, in bones — in 2 (4.6%) patients, in liver and bones — in 1 (2.3%) case. In the premenopausal group, lung metastases were found in 3 (6.97%) patients, in bones — in 4 (9.3%) cases, in liver – in 2 (4.65%) patients, in liver and bones — in 3 (6.97%) cases. A decrease in BMI in breast cancer patients leads to an improvement in the outcomes of the disease and to a decrease in the resistance of the tumor to chemotherapy.
5. Conclusions
- Our research work has shown that obesity is associated with an increased risk of her2/neu tumors and correlates with poor median metastasis-free survival among premenopausal patients with breast cancer. However, despite the fact that obesity was previously considered an unfavorable prognostic risk factor for triple-negative cancer in postmenopausal patients, there was no significant association between BMI and breast cancer in two groups. The results of our study show that high body weight reduces the sensitivity of tumor cells to chemotherapy. It is possible to achieve a better effect with chemotherapeutic treatment by recommending weight loss to patients with high BMI.Finally, there may be other risk factors for each immunohistochemical subtype that do not depend on the menopause period and BMI.A decrease of BMI in patients leads to better outcomes of the disease.The authors declare no conflict of interest. This study does not include the involvement of any budgetary, grant or other funds. The article is published for the first time and is part of a scientific work.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML