-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2023; 13(7): 949-954
doi:10.5923/j.ajmms.20231307.23
Received: Jul. 7, 2023; Accepted: Jul. 20, 2023; Published: Jul. 24, 2023

Acute Abdominal Surgical Diseases During the Covid-19 Pandemic: Clinical and Epidemiological Aspects and Issues of Optimizing Emergency Medicine (Literature Review)
D. G. Buribayev, D. B. Tulyaganov, Kh. E. Anvarov, A. O. Kurbanov
Republican Research Centre of Emergency Medicine, Tashkent Regional Branch of Republican Ambulance Center, Tashkent, Uzbekistan
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

December 2020 is considered the start of the spread of a new respiratory disease caused by the SARS-CoV-2 virus (severe acute respiratory syndrome 2 coronavirus). Since February 2020, the disease has been officially named COVID-19 by WHO. In spite of global efforts to limit the spread of the disease and quarantine measures, the infection has been recognized as a pandemic. By the end of March 2020, more than 750 thousand cases and more than 36 thousand deaths were registered [1]. SARS-CoV-2 is characterized by high transmissibility, while the spread occurs by airborne droplets and contact routes [2].
Keywords: COVID-19
Cite this paper: D. G. Buribayev, D. B. Tulyaganov, Kh. E. Anvarov, A. O. Kurbanov, Acute Abdominal Surgical Diseases During the Covid-19 Pandemic: Clinical and Epidemiological Aspects and Issues of Optimizing Emergency Medicine (Literature Review), American Journal of Medicine and Medical Sciences, Vol. 13 No. 7, 2023, pp. 949-954. doi: 10.5923/j.ajmms.20231307.23.
Article Outline
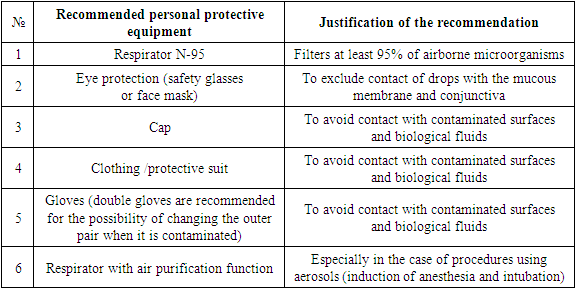
- Patients in critical condition needed ventilation support and hospitalization in intensive care units, which led to an overload of the healthcare system. WHO and the Centers for Disease Control and Prevention recommended avoiding getting into high-risk areas of infection and contact with infected patients [3]. However, signs of the disease in the majority of infected persons, who are potential sources of infection, are not revealed [4-6].Individual protective measures are recommended, such as frequent hand washing, the use of antiseptics, personal protective equipment, including masks, gloves, safety glasses. Many surgical departments, including operating rooms, have been transformed into additional intensive care units. Planned and non-oncological surgical interventions were postponed indefinitely. Medical and paramedical staff were relocated to infectious diseases departments. The pandemic required the introduction of new standards and algorithms for the reorganization of the healthcare system in general and the system of surgical care, in particular, in new epidemiological conditions. However, unified unambiguous algorithms of action in emergency situations, such as acute trauma or "acute abdomen", in a pandemic, have not been developed. During the COVID-19 pandemic, healthcare leaders have faced the challenge of converting the emergency medical service, including ambulances and intensive care units, to care for infectious patients while minimizing the risk of infection for staff, non-infectious patients, and in-hospital transmission. A surgeon receiving an emergency patient should take into account the limited availability of operating rooms (since many of them were converted to COVID-19 intensive care units), the limited availability of personal protective equipment, transfusion resources, as well as the risk of infection dissemination in the operating room. When planning a surgical intervention, the main decisive factors that a surgeon should consider are safety and prevention. It is necessary to formulate a solution to the following tasks for this purpose: minimizing the entry of viruses into the operating room; reducing the risk of equipment contamination with viral particles; minimizing the time spent in the operating room; the maximum reduction in the duration of hospitalization of patients with emergency surgical pathology.After a thorough clinical examination of the patient who applied for acute abdominal pain, and obtaining the results of laboratory and instrumental studies, the first question is whether it is possible to delay the surgical intervention (until the infection process in the patient is stopped or the risk of perioperative complications is reduced). If surgery is urgent and necessary (for life reasons), the surgeon must ensure that the appropriate operating room, equipment and personnel are available and functional. The golden rule is to attract the minimum necessary number of personnel, providing the maximum possible anti-infective protection [7]. The standard for the use of personal protective equipment is given in Table 1.
|
1. Emergency Surgery or Conservative Tactics?
- Early diagnosis, control of the spread of infection, adequate antimicrobial therapy, the use of intensive care methods is the basis of management tactics for patients with intra–abdominal infectious processes [8]. The main solution in this situation is whether the patient needs emergency surgery or non–surgical treatment is possible. The keys to the answer to this question are the clinical picture (signs of localized or diffuse peritonitis) and markers of the activity of a systemic inflammatory reaction (general blood test, pro-inflammatory markers). These parameters should be obtained as quickly as possible. Hemodynamic status is the key for evaluating perioperative risk. In addition, background and competing pathologies are used and various risk assessment scales are applied, such as the Scale of the American Society of Anesthesiologists (ASA), Alvarado score, severity of organ failure. Assessment of perioperative risk allows to weigh the risk-benefit ratio of emergency surgery.Intraabdominal infection can be classified as uncomplicated (without involvement of the peritoneum and signs of peritonitis) or complicated (with the development of local or diffuse peritonitis) [8]. In the case of an uncomplicated process, the choice in favor of non-surgical tactics seems to be more justified in most cases, according to the recommendations of the World Society of Emergency Surgery (WSES). In this situation, it is necessary to determine the tactics of observation – which clinical, laboratory and instrumental studies should be carried out in dynamics (every 12-24 hours) for full control of the patient's condition. If the patient has persistent abdominal pain, fever, signs of hemodynamic shock – such a patient is given indications for immediate surgical intervention.Appendicitis. Today, the gold standard of treatment for patients with acute appendicitis is laparoscopic appendectomy. During the COVID-19 pandemic, laparoscopic access was used with caution, since the virus could contaminate the peritoneum and be present in the pneumoperitoneum. In the case of uncomplicated appendicitis against the background of the use of antibiotics, non-surgical tactics can be an adequate choice. The Jerusalem guidelines of WSES 2020 recommend the use of non-surgical tactics in patients with uncomplicated appendicitis who wish to avoid surgery and are aware of the possible risk of recurrence of the disease, which can reach up to 39% [9]. Non-surgical management of patients does not increase the risk of perforation, in addition, most cases of relapse of the disease are also uncomplicated appendicitis [9], which makes this strategy (non-surgical management + antibiotic therapy) a safe alternative to surgery in a certain group of patients, especially during infectious pandemics, allowing to delay (if not neutralize) the need for surgical intervention by postponing it in case of relapse.Acute cholecystitis. Laparoscopic cholecystectomy is the method of choice for the treatment of acute cholecystitis [10]. In particular, early cholecystectomy is preferable to late and delayed. Many randomized controlled trials have shown that early cholecystectomy is associated with a shorter hospital period, with no difference in the risk of complications and conversion to laparotomy. However, the recommendations for early intervention underwent rethinking during the COVID-19 pandemic due to the limitations imposed by the infection. In this situation, it is necessary to consider the possibility of delayed intervention against the background of therapy with antibacterial drugs and analgesics. Patients in critical condition may undergo percutaneous cholecystectomy. In fact, in 2016, the WSES guidelines do not recommend percutaneous cholecystectomy as an alternative to laparoscopic access, except in patients with high perioperative risk, due to the high risk of mortality and complications [10]. A recent CHOCOLATE study confirmed these data and was prematurely completed due to significantly unfavorable results in a group of patients with percutaneous cholecystectomy [11].Diverticulitis. According to the WSES guidelines, the method of choice in the treatment of uncomplicated acute diverticulitis is a non-surgical tactic with the use of intravenous antibiotic therapy followed by a transition to oral forms [12]. Patients with generalized peritonitis need emergency surgery. Patients with Diverticulitis of class I and II according to the Hinchey classification require percutaneous drainage of the abscess against the background of antimicrobial therapy, if the abscess with a diameter of 4 cm or more is visualized on a computed tomogram of the abdominal cavity. If percutaneous drainage is not possible, antibiotic therapy is recommended for the patient. Indications for surgical treatment are the presence of sepsis signs or hemodynamic shock, as well as the ineffectiveness of a non-surgical strategy.Patients with signs of free gas in the abdominal cavity and peritonitis require surgical intervention. In this situation, the possible options are [12]: Hartmann's procedure in case of diffuse peritonitis in critically ill patients or in patients with multiple comorbidities that aggravate the patient's somatic status; primary resection with anastomosis or stoma in clinically stable patients.Emergency laparoscopic sigmoidectomy should be avoided due to the risk of aerosolization, especially if a long duration of surgery is expected.Obstruction and perforation of the colon. According to the guidelines of the World Society of Emergency Surgery for left-sided colon obstruction, the procedure of choice is loop colostomy (short duration of surgery) or Hartmann's procedure [13]. The advantage of Hartmann's procedure over a simple colostomy is a shorter hospital stay and simultaneous correction of the disease. On the other hand, a loop colostomy can serve as a temporary "bridge" before radical correction. In the conditions of a pandemic, an individual treatment plan for such patients depends both on the clinical status of the patient and on the resources and surgical capabilities available in the hospital.In general, it is recommended to use a loop colostomy for patients with unresectable colon tumors and for patients with high perioperative and anesthetic risk. Colon stenting may also be a treatment option, but endoscopic procedures in a pandemic contribute to the dissemination of the virus and are recommended only for patients who have a high surgical or anesthetic risk [13].Colon resection and primary anastomosis with or without ileostomy is the preferred choice in the case of uncomplicated colon obstruction by a malignant tumor. However, this option of surgery is associated with an increase in the duration of surgery and a corresponding increase in the risk of the spread of the COVID-19 virus. Hartmann's procedure is recommended for patients with high perioperative risk. Total colectomy is not recommended in the absence of dilatation of the caecum, ischemia of the colon, synchronous cancer of the right half of the colon [13]. In the case of obstructive right-sided colon cancer, right-sided colectomy with primary anastomosis is the best surgical option. If a primary anastomosis is considered risky, an end ileostomy with a colonic fistula may be an alternative. [13]. In the case of unresectable cancer of the right side of the colon, a side-to-side ileotransverse anastomosis can be performed as a bypass or a loop ileostomy can be performed [13].Adhesive obstruction of the small intestine. Even during the COVID-19 pandemic, in a situation of small intestinal obstruction, non-operative management of patients in the absence of signs of peritonitis, strangulation intestinal obstruction or intestinal ischemia became the option of choice. The efficiency of such tactics is 70-90%. Most authors believe that a wait-and-see tactic is justified and safe for 72 hours [14]. Strangulated abdominal hernia. If strangulation ileus is suspected, emergency surgery is recommended to prevent intestinal ischemia [15]. If an inguinal hernia is incarcerated, intervention using local anesthesia (in the absence of intestinal gangrene) is possible to reduce the risk of spreading the COVID-19 virus in the operating room.Acute pancreatitis during the COVID-19 pandemic. The most characteristic clinical manifestation of a new coronavirus infection is a respiratory syndrome, in particular dyspnoea, cough, dry throat [16]. The second most common syndrome is gastrointestinal, including stomach paresis, gastritis, enteritis, colitis and pancreatitis. Involvement of the gastrointestinal tract (GIT) may be associated with the expression of angiotensin type II receptors by epithelial and exocrine cells of the intestinal tube [17-18].Pancreatic cells also express type 2 angiotensin receptors, even in greater numbers than alveocytes [19]. SARS-CoV-2 RNA has been identified in the gastrointestinal tract, including pancreatic cells by many researchers [20-22]. A lot of data has also been published showing the development of pancreatitis in patients with COVID-19 even before the onset of respiratory symptoms [23-26]. There are 2 main hypotheses for the development of pancreatitis in patients with COVID-19: direct cytotoxic viral injury to cells or delayed injury due to an immune response [27-29]. A meta-review [4] describes data from 40 studies, including 46 patients aged 24-87 years, who met the diagnosis of acute pancreatitis according to the Atlanta criteria system (2 out of 3: abdominal pain - 63% of patients, amylase or lipase activity 3 times exceeding the upper limit of the norm - 92% of patients, characteristic data of visual examination methods - 82.6%) [30] in the absence of precipitating factors other than COVID-19 (cholelithiasis, hypertriglyceridemia, alcohol intoxication). Expression of angiotensin type 2 receptors makes pancreatic cells a potential target for SARS-CoV-2 [31]. The clinical picture of acute abdominal surgical pathology in patients with COVID-19 may be pathogenetically not associated with a viral infection, but be a coincidence, causing competing comorbidity [32].
2. Choice of Access: Laparoscopy or Laparotomy?
- Patients with inefficiency of non-surgical tactics or with signs of unstable hemodynamics are candidates for surgical intervention. Traditionally, the advantage of laparoscopic access is recognized in emergency surgery. Since this tactic helps to reduce the duration of hospital stay, less severity of pain symptoms, less pronounced violations of the function of external respiration and rapid postoperative recovery [33]. Unambiguous research results confirming the spread of the virus in the operating room during laparoscopic interventions have not been published. It is known that SARS and SARS-CoV-2 are predominantly spread by direct entry of infected secretions, but indirect spread by environmental contamination is also possible, especially for nosocomial spread of the pathogen [33-35]. Van Doremalen N, et al [35] demonstrated that SARS-CoV-2 is more persistent in aerosols and on various surfaces (plastic and metal) than SARS-CoV-1 and remains viable in aerosols for hours and on surfaces for days.According to the above mentioned data, recommendations were issued in the UK (April 7, 2020), which suggest using laparoscopic access only for some patients whose risk of spreading the virus is significantly lower than the perioperative risk [36]. Conversely, some authors believe that the laparoscopic procedure reduces the risk of virus spread compared to open surgery because the abdominal cavity remains closed, although it is recommended to filter the surgical gas at the laparoscopic exit port to reduce the risk of operating room contamination [37]. In general, based on the literature data, if a laparoscopic approach is chosen, it is necessary to control the leakage of gas into the air of the operating room. For this purpose, constant-pressure insufflation and aspiration techniques should be used, including the closed loop option or the use of a water lock for the outflow port. [38-40]. Before accessing the outflow port, the gas must be turned off and the pneumoperitoneum reduced to negative [38]. It is necessary to remove residual carbon dioxide from the abdominal cavity before removing the trocar.
3. Strategy of Controlled-Risk Surgery and Open-Access Abdominal Surgery
- Risk-controlled surgery strategy is used in hemodynamically unstable patients [41]. These patients, as well as patients who underwent open abdominal surgery, need to be hospitalized in the intensive care unit. However, in the conditions of a pandemic, there is a shortage of available beds in the intensive care unit, which serves as an additional argument in favor of considering the option of non-surgical management of patients with acute abdominal pathology.
4. Preparation of the Operating Room
- In a pandemic, it is important to determine the in-hospital route for patients with suspected or confirmed COVID-19 [42]. Hospital staff who are in contact with patients must comply with sanitary and hygienic rules and use personal protective equipment, avoid contact with mucous membranes (eyes, nose, mouth) [42-43]. All surgical procedures should be performed in operating rooms with reduced air pressure in order to decrease the risk of infection spreading outside the operating unit. In the case of an operating room with positive pressure, frequent air exchange is required (25 times per hour) [42-44].It is necessary to limit the number of people involved in the surgery. All team members should be available for quick contact in case of suspected infection. The entrance to the operating unit must be closed during the entire procedure, the movement of personnel to and from the operating unit is restricted [45-47]. It should also be limited to the minimum required number of pieces of equipment and packages of medicines in the operating room. If it becomes necessary to use additional equipment/consumables, outside the operating unit, a hospital employee should be ready to deliver the necessary materials on demand. The remaining medicines and unused consumables after the procedure should be cleaned according to sanitary requirements or disposed of to limit the spread of infection. The surfaces of anesthetic monitors, computer systems, ultrasound machines should be covered with plastic covers to reduce contamination and ensure effective sanitation [42-44].Preparation of the patient for surgery, preoperative examination, induction of anesthesia should be carried out directly in the operating room, as well as postoperative awakening from anesthesia, to exclude virus contamination of the additional room. The operating team must use additional protective equipment: a respirator with an air purification function, high shoe covers, a waterproof protective suit, protective glasses or a face mask.After the end of the surgery, personnel who are not involved in the postoperative management of the patient should go to the preoperative room, all personal protective equipment used is placed in a container for infected material, it is necessary to take a shower, change clothes before returning to routine work. The operating room must be processed immediately after the end of the procedure. It is recommended to spray hydrogen peroxide to decontaminate the room. SARS-CoV-2 on surfaces is effectively inactivated with 62-71% ethanol, 0.5% hydrogen peroxide, or 0.1% sodium hydrochloride with a 1-minute exposure. Other biocidal agents such as 0.05-0.2% benzalkonium chloride or 0.02% chlorhexidine digluconate are less effective [48].Thus, due to the high contagiousness of SARS-CoV-2 and the severity of the pathology it causes, the healthcare system is overburdened and unable to function according to standard protocols. In fact, COVID-19 was compared with a global natural or man-made disaster in terms of the load on the public health system, both in terms of the number of victims and the severity of their condition [45]. Surgical departments were converted into infectious departments and intensive care units since the beginning of the pandemic. Non-emergency and non-oncological interventions were postponed, the staff of the surgical departments, operating units, surgical intensive care units were sent to the disposal of the infectious diseases service, the equipment was used to provide the necessary procedures (for example, ventilation support) to patients with COVID-induced pneumonia. Patients requiring anti-cancer procedures were placed at the top of the waiting list. Patients who needed postoperative intensive care were transferred to specially designated government centers [47].In order to prevent the spread of infection of patients admitted with emergency surgical pathology, it was recommended to divide medical institutions into COVID-19 positive and COVID-19 negative. In each COVID-19 positive hospital, clear coordination between the surgeon and the anesthesiology team was required to make optimal use of available resources and minimize the risk of infection spreading. There was no consensus on isolating staff working in COVID-19 positive clinics. Patients who have not been tested for COVID-19 (COVID-19 undetermined), including those without respiratory symptoms, should have been considered potentially infected and conducted taking into account their potentially positive status. The progressive increase in the number of infected medical personnel forced to tighten the methods of control and use of personal protective equipment [46].The authors declare no conflict of interest. This study does not include the involvement of any budgetary, grant or other funds. The article is published for the first time and is part of a scientific work.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML