-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2023; 13(7): 937-941
doi:10.5923/j.ajmms.20231307.20
Received: Jun. 12, 2023; Accepted: Jul. 3, 2023; Published: Jul. 13, 2023

Malignant External Otitis Consequences as Conductive Hearing Loss and New Diagnostic Methods
U. I. Nurov, S. SH. Fayziyev
Bukhara Medical Institue after name of Abu Ali Ibn Sina
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Purpose: The progression of the otitic infectious process toward diseases of particular severity is often unpredictable, just as it is challenging to manage the patient over time, even after the apparent resolution of the disease. We aim to define a radiological reading key that allows us to correctly and promptly treat the disease, avoiding the possible severe complications. Methods: We conducted a retrospective study of 13 cases of basal cranial osteomyelitis (SBO) due to malignant external otitis, by the ENT Department of the Bukhara medical Institute. Through a standardized approach and following the latest guidelines, we have evaluated all patients performing a standardized and personalized radiological protocol according to the stage of the patient’s pathology and modulating the treatment consequently. Results: Clinical signs have been observed such as otorrhea (100%), otalgia in 13/13 patients (100%), granulations in external auditory canal (100%), preauricular cellulitis in 9/13 patients (69%) headache 6/13 cases (46%), dysphonia 4/13 cases (31%). HRCT of the temporal bone proved useful in identifying even minimal bone lesions in 13/13 (100%) while improving MRI in vascular and nervous involvement, although in 1/13 patient with nerve palsy clinical symptomatology preceded radiological evidence. The 99mTc 3-phase planar bone scintigraphy was positive for SBO in 9/13 cases (69%) during the initial phase and, in 100% of the cases in images delayed to 2-3 hours. Subsequent checks up to 1 year, using the Ga 67 scintigraphy, excluded the presence of recurrences in 100% of patients. Conclusion: The osteomyelitis of the base of the skull is a severe complication of malignant external otitis, often not always easily diagnosed. Recurrence can occur up to 1 year after stopping therapy. Imaging techniques such as Tc and MRI are relevant for the initial diagnostic approach and the staging of the pathology and its complications. Nuclear medicine imaging plays a fundamental role in the evaluation of related osteoblastic activity, especially in the remission phase of the disease.
Keywords: Necrotizing otitis externa, Skull base, Radiological findings, Osteomyelitis
Cite this paper: U. I. Nurov, S. SH. Fayziyev, Malignant External Otitis Consequences as Conductive Hearing Loss and New Diagnostic Methods, American Journal of Medicine and Medical Sciences, Vol. 13 No. 7, 2023, pp. 937-941. doi: 10.5923/j.ajmms.20231307.20.
1. Introduction
- Necrotizing “malignant” external otitis (MEO) is a rare necrotizing infection caused by Pseudomonas Aeruginosa, which spreads from the squamous epithelium of the external auditory canal to the surrounding tissues, first described by Meltzer in 1959. [1] Patients with otalgia and otorrhea persistent despite ambulatory therapy for several weeks. Typically, the infection spreads from the EAC through the base of the skull, causing bone erosion with the development of soft tissue granulomas. [2,3] The treatment of the disease provides an initial phase of ggressive pharmacological treatment.Radiodiagnostic imaging methods perform a fundamental role in the global evaluation of prognosis and the treatment of malignant external otitis and in particular in its complications of the skull base. The imaging techniques related to the initial diagnostic approach are CT and MRI, with the first one, to evaluate erosion and demineralization. In contrast, the details of the soft tissues and the base of the skull are involved using the second one. Other diagnostic methods support the diagnosis of SBC are Tc-99 m-MDP, related to the increase in osteoblastic activity of the disease. [4]The natural course of the otitic infectious process towards diseases of particular gravity is often unpredictable, putting a strain on the choice of the clinician who faces the pathology, among other things of rare frequency. Moreover, even the posttherapeutic management of the patient in apparent healing is difficult, considering the insidious recurrences. Our goal is to define a radiological reading key allowing us to treat the disease correctly and promptly, avoiding possible and severe complications. Through the analysis of radiological criteria correlated with clinical data, it is indeed possible to define the correct approach of the otitic patient.
2. Material and Methods
- We conducted a retrospective study of 13 cases of cranial osteomyelitis (SBO) due to malignant external otitis, from January 2020 to December 2022, at the ENT Department of the Bukhara medical institute. The average age range was 54 to 82 years, composed of 10/13 men (77%) and 3/13 women (23%). Regarding risk factors, 9/13 patients (69%) had diabetes mellitus, 3/13 patients (23%) had undergone chemotherapy and, 1 patient (8%) underwent bone marrow transplantation. All included patients presented characteristic symptomatology for an average time of about 2 months + 1, not responding to the conventional antibiotic therapy. The age, sex and medical history of these patients have been recorded. Initially, the patients underwent a thorough clinical examination, demonstrating clinical features such as deep pain (temporal, parietal, retro auricular, retroorbital), headache, intermittent otorrhea and spiking fever. Moreover, were reported preauricular cellulitis and mastoiditis, hearing floor granulation and cranial nerve involvements. The evaluation of the systemic inflammatory status was performed utilizing hematological examinations (routine blood tests, HGT analysis, VES monitoring, and PCR). The auricular swab and biopsies from the granulation tissues were analyzed by histopathological examination (HPE). An immunoabsorbent test linked to the enzyme for human immunodeficiency virus (HIV) was also performed to analyze the status of patients’ immune systems.In particular, in each subject at the end of the clinical evaluation, a Radiodiagnostic protocol was performed consisting of high-resolution computed tomography (HRCT) of the temporal bone (axial and coronal sections) and Magnetic Resonance Imaging (MRI) (axial and coronal sections).While the Tc-99 m-MDP test was performed to strengthen the diagnosis of the osteomyelitis process immediately after the 2 procedures mentioned above, the Ga 67 scintigraphy was executed within 1 month of stopping antibiotic therapy in all cases. Besides, each patient was evaluated by 2 independent radiologists with at least 15 years of experience in the Head and Neck district.The Positron Emission Tomography (PET) was performed in 2 patients as an alternative follow-up method.The final resolution of the infectious process was expressed only after 12 months of wellbeing from the end of the treatment with concomitant compliance with the established Radiodiagnostic criteria with the integration of clinical symptoms and laboratory tests.
3. Results
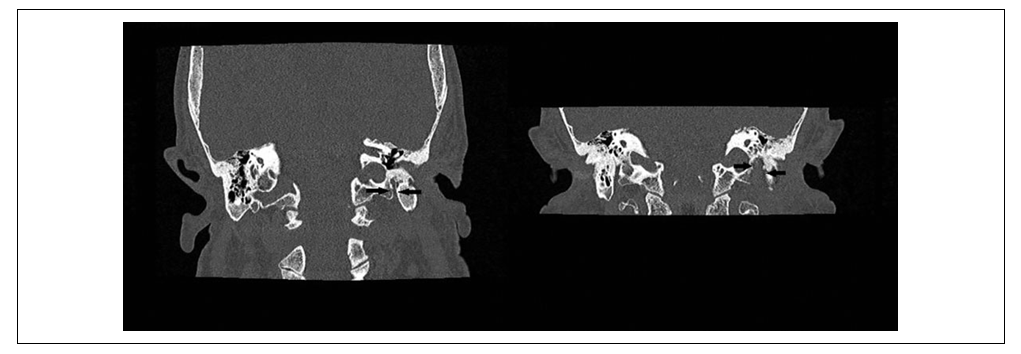
- The most common clinical features observed were: otorrhea (100%); otalgia in 13/13 patients (100%); granulations in the EAC (100%); preauricular cellulitis in 9/13 patients (69%) headache 6/13 cases (46%); dysphonia 4/13 cases (31%).All patients had cranial neuropathy at the time of clinical diagnosis. Facial nerve palsy was observed in 12/13 cases (92%): mainly associated with other cranial nerves paralysis 7/13 (54%), the isolated involvement resulted only in 5/13 (38%).Moreover, 3/13 patients presented IX, X, XI and XII cranial nerve palsy (23%) while 1/13(8%) patient, who was subjected to chemotherapy, showed isolated VI CN paralysis.Furthermore, 1/13 (8%) patient who performed Bone Marrow transplantation, showed an associated involvement of the VI and VII CN.The involvement of the temporomandibular joint was observed in 3/13 cases (23%). Only 1/13 patient (8%), presenting a case of paralysis of the VII, developed meningitis, antibiotics bilateral cocleopathy, arteritis and thrombosis of the internal carotid.HRCT of the temporal bone showed in all cases common findings such as soft tissue density in the EAC and the middle ear, the opacification of mastoid cells of air with cortical bone erosion and bone seizure in all patients (100%) (Figure 1). Moreover, HRCT revealed in 2/13 patients (23%) the erosion of the EAC floor extending anteriorly in the temporomandibular joint, while in 1/3 patient with a positive temporomandibular clinic did not demonstrate visible lesions.
4. Discussion
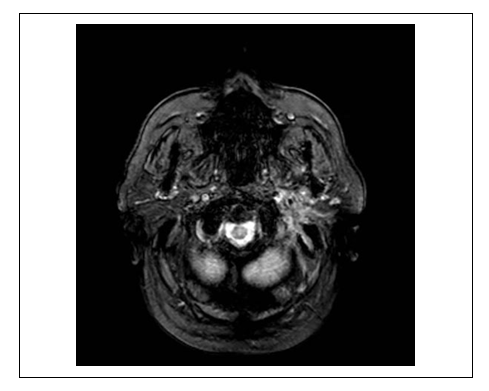
- Malignant or necrotizing otitis externa (MOE) was first described by Meltzer in 1959 and formally clinically defined by Chandler in 1968. [5]MOE occurs predominantly in elderly diabetics or immunocompromised, and the most common pathogen is Pseudomonas Aeruginosa, but cases of Staphylococcus, Candida and Aspergillus have also been reported. [6]Several nosological criteria have emerged in the literature to confirm the diagnosis of malignant external otitis. [7-9] Levenson’s criteria are used for diagnosis of necrotizing otitis externa, including refractory otitis externa, severe nocturnal otalgia, and purulent otorrhea associated with Pseudomonas infection and granulation of the EAC in an immunocompromised or diabetic patient and positive temporal bone scanning with Tc99. [10]Although in most cases the patient has a disproportionate otalgia compared to the clinical picture, nonspecific symptomatology characterized, for example, by persistent headache and development of cranial neuropathy is possible.MOE is the end stage of a severe infection that originates from the EAC and could progress through cellulitis to chondritis, osteitis and at the end of osteomyelitis. Once periostitis develops, the infection progresses rapidly across the skull base, replacing the progressively compact bone with granulation tissue. As a result, facial and other cranial nerve palsies can occur. [3,11] Parotitis and trismus secondary to masseter myositis and involvement of the temporomandibular joint can also develop in SBO. [11,12]Diffusion along the temporal bone through the fissure of Santorini commonly involves the styloid mastoid foramen (containing the facial nerve) and the jugular foramen (containing the glossopharyngeal, vagal and accessory nerves). The infectious process, from the fissure of Santorini, spreads to retro condyloid fat, parapharyngeal fat, temporomandibular joint, and masticatory muscles.Prasad et al. divided temporal bone osteomyelitis into these 3 categories to define the spread of disease: osteomyelitis of the lateral temporal bone (bone involvement of the mastoid and middle ear cleft), medial temporal bone osteomyelitis (bone involvement of the otic capsule, petrous apex, and clivus) and pan temporal osteomyelitis (showing features of both mastoid/ middle ear cleft and petrous involvement). [2,13]Lesser et al. related the onset of disease with malignant external otitis, thus describing 3 different types of skull base osteomyelitis. In the first case, the necrotizing external otitis, detected at the clinical and diagnostic examination, extends to the skull base. The development of osteomyelitis represents another possibility after the resolution of the necrotizing external otitis. Finally, osteomyelitis can occur primary without any apparent procedural lateral infection. [14]Moreover, Kwon et al. described 4 spreading patterns of the soft tissue extension: medial, anterior, crossed and intracranial spreading. In addition to the patterns mentioned above, there can be intravascular involvement. [12]The diagnostic approach of osteomyelitis at the base of the skull can be a challenge, considering that the diffusion model can be nonspecific for an infectious process.Despite the characteristic pain symptoms and the involvement of the cranial nerves, the symptoms and signs of a severe infection can be almost absent. [15]For example, the absence of infectious symptoms such as fever, pain, swelling, and increased ESR and white blood cell counts may be absent in immunocompromised patients, with possible diagnostic error.Moreover, the involvement of the cranial nerves of the advanced SBO can be associated with difficulties with swallowing and paralysis of the facial nerve, pathological signs commonly associated with a neoplastic process of the base of the skull. [16]Although the osteomyelitis of the cranial base presents local edema of the tissue and extensive diffuse bone destruction at radio diagnostic examinations, imaging can sometimes be highly suggestive but not specific to the pathology as mentioned earlier. [17]Even after the conclusion of followup, patients who have developed severe complications, such as cranial nerve involvement, do not have complete recovery of the primary function. Differential diagnosis of the disease should consider Squamous cell carcinoma of the head and neck, which may also involve soft tissues of the calvarium and preclival and destruction of the base of the skull. Other differential diagnoses are nasopharyngeal carcinoma, multiple myeloma, lymphoma and metastatic processes. [18-21]Among the imaging methods available to date, computed tomography (CT) is the most commonly used for the initial approach to the disease in question. It allows to quickly and relatively easily evaluate both the involvement of soft tissues and the degree of demineralization of the mastoid bone. [14]Through the use of ultra-thin high-resolution Tc, the evaluation of bone erosion is optimal, but this is not necessarily present, especially in the initial stages of the pathology (Figure 1).The strength of this modality is the evaluation of bone erosion and demineralization, in particular with the use of high-resolution (ultra) thin CT on several levels.Since the infectious outbreak originates from an initial process of the external ear, the CT scan can show either the classic finding of edema of the external auditory canal with the inflammation of the surrounding adipose tissue or very slight differences of soft tissues only appreciable comparing them with the contralateral ear. [22]Indeed, a frequent consequence is the anterior development of the pathology up to the temporal mandibular articulation with the presence of associated destructive phenomena. However, bone involvement is often a late phenomenon, common also to other inflammatory or neoplastic diseases.The diagnostic method must also study the extension to the skull of the base of the foramina, in particular, the jugular and stylomastoid foramen, frequently involved.In this regard, through the analysis of the subtemporal fat, and parapharyngeal fat plans, it allows us to evaluate the median diffusion of the nasopharynx through the Eustachian tube. However, these plans are more difficult to study than the masticatory or retromandibular one.Magnetic resonance imaging is the superior imaging technique for anatomical evaluation of soft tissue components compared to CT, considering, for example, signs such as thrombosis and intracranial spread (Figure 2). [17]The T1-w sequences allow identifying fat planes adjacent to the base of the skull, the sub temporal region, the space of the mastoid. Even the muscles affected by the inflammatory process demonstrate an altered signal in T1-w, such as arterial and venous vascular involvement. Although it is not possible to detect alterations of the cortical bone, indirect signs are represented by an abnormal signal of the bone marrow (Figure 3).
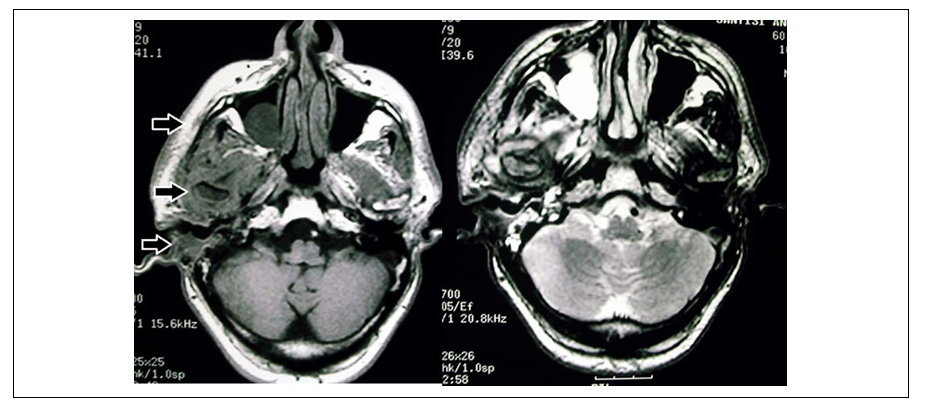 | Figure 3. MRI scan T1w / T2: wide diffusion of the infectious process in the soft tissues starting from CUE. The region of the temporomandibular joint appears to be infiltrated |
5. Conclusions
- The osteomyelitis of the base of the skull is a severe complication of malignant external otitis, characterized by a symptomatologic pattern that is often but not always clear and easily diagnosed. Therefore, considering the often shaded and subtle interpretation clinic, the correct interpretation of radiological examinations is essential, identifying critical points in the eva luation of the pathology.The major complication of MOE is the involvement of the skull base, with subsequent paralysis of the cranial nerves and hearing loss. Recurrences of MOE can occur up to 1 year after cessation of therapy, so a patient must be considered cured until that period has elapsed. Furthermore, the pathology is insidious due to the lack of correlation between the severity of this and the associated clinical-symptomatologic picture, making imaging the only way to avoid severe complications. Numerous new high-resolution and highly useful methods are described in the literature, but the relatively low diffusion of these technol ogies in primary and first-level hospitals must be considered.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML