-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2023; 13(7): 924-927
doi:10.5923/j.ajmms.20231307.18
Received: Jun. 11, 2023; Accepted: Jun. 30, 2023; Published: Jul. 12, 2023

Effect of the New Drug "Rheoambrasol" on Lipid Peroxidation and Antioxidant System of the Liver in Hypoxia Caused by Hemorrhagic Shock
Jamoliddin Khujakhmedov1, Larisa Shevchenko2, Khamid Karimov2
1"GenoTexnologiya" Molecular Genetic Laboratory, Tashkent, Uzbekistan
2Department of Molecular Medicine and Cellular Technologies, Republican Specialized Scientific-Practical Medical Center of Hematology MoH RUz, Tashkent, Uzbekistan
Correspondence to: Larisa Shevchenko, Department of Molecular Medicine and Cellular Technologies, Republican Specialized Scientific-Practical Medical Center of Hematology MoH RUz, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The aim of the work is to study the effect of the infusion medical drug Rheoambrasol on the content of hypoxia-inducible factor HIF-1α, lipid peroxidation and antioxidant system in the liver in hemorrhagic shock. The work was performed on a model of hemorrhagic shock (SH) on 40 male Chinchilla rabbits. The blood content of hypoxia factor (HIF-1α), lipid peroxidation, state of antioxidant system (activity of catalase, superoxide dismutase, glutathione reductase, glutathione peroxidase) and animal survival were studied in dynamics. The results of the study showed that the new blood substitute Rheoambrasol has an antihypoxant effect, reducing the level of hypoxia-inducible factor HIF-1α in hemorrhagic shock by 5.1 times (p<0.05) and increasing animal survival rate in hemorrhagic shock. The use of a new infusion drug Rheoambrasol in hemorrhagic shock helps to suppress the intensity of LPO processes and activates the activity of AOS enzymes in the liver. Rheoambrasol in hemorrhagic shock was effective in protecting the liver from free-radical oxidation and exceeds “Rheopolyglukin” in effectiveness.
Keywords: Hypoxia, Hemorrhagic shock, Antihypoxant, LPO, AOS, Liver, HIF-1α
Cite this paper: Jamoliddin Khujakhmedov, Larisa Shevchenko, Khamid Karimov, Effect of the New Drug "Rheoambrasol" on Lipid Peroxidation and Antioxidant System of the Liver in Hypoxia Caused by Hemorrhagic Shock, American Journal of Medicine and Medical Sciences, Vol. 13 No. 7, 2023, pp. 924-927. doi: 10.5923/j.ajmms.20231307.18.
Article Outline
1. Introduction
- In the multifaceted pathogenesis of hemorrhagic shock, along with disorders of cardiovascular system, microcirculation, acid-base status, water-electrolyte balance, hemocoagulation and blood rheology, a major role is played by hypoxia of complex genesis, which contributes to disruption of energy exchange, activation of lipid peroxidation and complex suppression of antioxidant defense system [1,3]. Recently, much attention has been paid to the development of effective antihypoxic agents of metabolic type of action for the treatment of extreme conditions [6,10,13]. One such remedy is a new antihypoxic remedy "Rheoambrasol", containing a complex of polysaccharide derived from local plant raw materials and metabolite of the Krebs cycle, capable of protecting cells from free radical oxidation and restore the disturbed energy metabolism in cells under hypoxic conditions [11].
2. Aim of the Study
- The aim of the work is to study the effect of “Rheoambrasol” on the content of hypoxia-inducible factor HIF-1α, lipid peroxidation and antioxidant system in the liver in hemorrhagic shock.
2.1. Materials and Methods
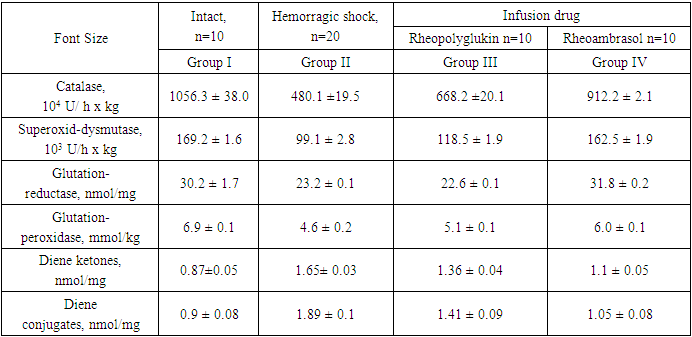
- The work was performed on a model of hemorrhagic shock on 40 male rabbits weighing 2.2 ± 0.2 kg.Animals were divided into 4 groups:Ι - intact (n=20),ΙΙ - after hemorrhagic shock (n=16),ΙΙΙ - after hemorrhagic shock and subsequent infusion of “Rheopoliglyukin” (n=7),ΙV - after hemorrhagic shock and subsequent infusion of the new drug “Rheoambrasol” (n=8).Hemorrhagic shock was modeled by fractional bloodletting from the femoral artery for 1 hour until the blood pressure dropped to 40 mm Hg. Art., followed by reinfusion of 1/3 of the released blood [11]. The total amount of blood released into the reservoir was 28.0±2.6 ml/kg. 1 hour after hemorrhagic shock, the animals received a single infusion of "Rheoambrasol" in the experimental group IV and the drug "Rheopoliglyukin" in group III (comparison) in a volume of 40 ml per 1 kg of body weight. 1a). In dynamics, the content of hypoxia factor (HIF-1α) in the blood was studied, which was studied by the method of enzyme-linked immunosorbent assay (ELISA) using kits. The measurements were carried out on an MR 96 analyzer (Myndray, China).The content of MDA, diene ketones, diene conjugates, glutathione reductase (GR), glutathione peroxidase (GPO), activity of superoxide dismutase (SOD) and catalase was determined in the liver homogenate. MDA level was determined according to the method of L.I. Andreeva et al. using “TBK-AGAT” kits (AGAT). The products were calculated using the molar extinction coefficient and expressed in nmol/mg. Diene conjugates and diene ketones were determined according to the method of Titeeva G.R. and Korovina N.N. [3,7,15]The activity of AOS enzymes was expressed in units (units) per gram of raw liver weight, and as specific activity [4]. The activity of catalase was determined by the method of M.A. Korolyuk et al. (1998), the principle of which is based on the ability of H2O2 to form a stable coloured complex with molybdenum salts. Measurements were performed at a wavelength of 410 nm [8]. Catalase activity of the samples under study was determined by spectrophotometer and expressed in mmol/min/g protein.SOD activity was determined according to the method of V.G. Mkhitryan et al. (1978), which was calculated by the percentage of inhibition (T%) of tetrazolium blue reduction in alkaline medium. SOD activity was expressed in units/min x mg of protein [5]. Superoxide dismutase (SOD) activity was expressed in mmol/min/mg protein [2,9]. Purified SOD preparation (ICN Biomedicals, USA) was used as a standard [14].The activity of GPO was determined by the accumulation of oxidized glutathione (GSSG) as a result of lipoperoxide decomposition. The activity of the enzyme was expressed in units/min x mgHb per min. The activity of erythrocyte glutathione reductase (GR) was determined in the reaction medium of phosphate buffer at 340 nm and by NADPH*H decrease and expressed in μM NADPH2/min x g Hb (Vlasova S.N. et al., 1990) [12].All measurements were made on a spectrophotometer "UNICO" (USA).Statistical processing of the obtained data was performed using Student's test using "Microsoft Office Excel" and "Biostatistics 4.03" programs. The criterion of statistical reliability was p<0.05 m.
3. Results of the Study
- The results of the study obtained in hemorrhagic shock showed that the condition of experimental rabbits, was extremely severe, as evidenced by a sharp, statistically significant increase in the content of the hypoxia factor (HIF-1α), by 6.8 times (p<0.001) (Fig. 1). Increased level of HIF-1α indicates the development of hypoxia and disorders of hepatocyte oxygenation in hemorrhagic shock.
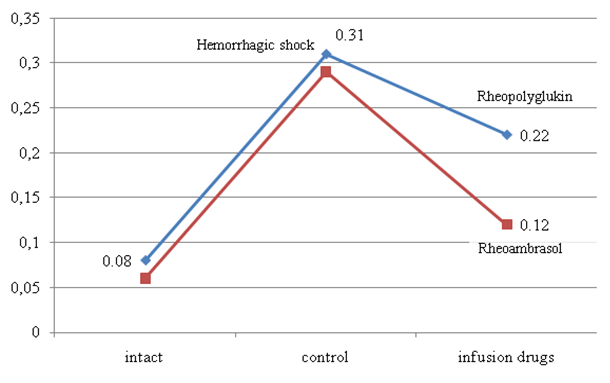 | Figure 1. Changes in the concentration of hypoxia-inducible factor (HIF-1α) in rabbits after hemorrhagic shock and infusion of infusion drugs |
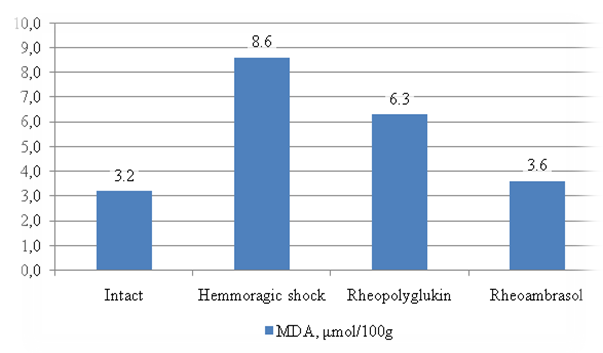 | Figure 2. Changes in MDA in the liver during hemorrhagic shock (HS) and after infusion of infusion drugs |
|
4. Conclusions
- The new drug "Rheoambrasol" has an antihypoxant effect, reducing the level of hypoxia-inducible factor HIF-1α by 5.1 times (p1<0.05) and increases the survival rate of animals with hemorrhagic shock.The use of a new drug "Rheoambrasol" in hemorrhagic shock contributes to the suppression of the intensity of lipid peroxidation processes and activates the activity of AOS enzymes in the liver."Rheoambrasol" in hemorrhagic shock turned out to be an effective means of protecting the liver from free radical oxidation, surpassing "Rheopolyglukin" in its effectiveness.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML