-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2023; 13(7): 892-893
doi:10.5923/j.ajmms.20231307.09
Received: Jun. 3, 2023; Accepted: Jun. 25, 2023; Published: Jul. 8, 2023

Modern Methods of Treatment of Immune Thrombocytopenia with Recombinant Human Thrombopoietin - Eltrombopag
Fayzullaeva N. I.1, Makhmudova A. D.1, Fayzullaev B. R.2, Ruzmetova F. A.2
1Republican Specialized Scientific Practical Medical Center of Hematology, Tashkent, Uzbekistan
2Urgench Branch of Tashkent Medical Academy, Urgench, Uzbekistan
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

This research aims to determine the effectiveness of Eltrombopag in the treatment of immune thrombocytopenia. Materials and methods. Eltrombopag was administered to a group of 30 patients with immune thrombocytopenia, the results were studied every 7 days. When determining the hemogram on the Sysmex apparatus, 79% of patients showed an increase in platelets, which made it possible to bring patients out of critical condition. It has also been established that the duration of the disease may affect the results obtained.
Keywords: Eltrombopag, Thrombocytopenia, Glucocorticosteroids, Romiplostim, Eltrombopag
Cite this paper: Fayzullaeva N. I., Makhmudova A. D., Fayzullaev B. R., Ruzmetova F. A., Modern Methods of Treatment of Immune Thrombocytopenia with Recombinant Human Thrombopoietin - Eltrombopag, American Journal of Medicine and Medical Sciences, Vol. 13 No. 7, 2023, pp. 892-893. doi: 10.5923/j.ajmms.20231307.09.
1. Introduction
- Immune thrombocytopenia - is an autoimmune disease caused by antiplatelet antibodies (AT antibodies) and / or circulating immune complexes (CIC), which usually affect the membrane glycoprotein structures of platelets and cause their destruction by cells of the reticuloendothelial system. In about half of the cases, antiplatelet antibodies are detected in the blood, for example, to glycoprotein IIb-IIIa and Ib -IX. Platelet destruction in ITP is caused by certain plasma factors, later called antiplatelet factors.Treatment of ITP is usually initiated when platelets fall below 30x10 9 /l or when a clinically significant hemorrhagic syndrome occurs, regardless of platelet levels. Systemic glucocorticosteroids (GCS) are the standard first-line therapy. In a randomized trial in 2015, splenectomy, rituximab, fostamatinib, and specific thrombopoietin receptor agonists (romiplostim, eltrombopag) are used in cases of insufficient effectiveness of corticosteroids. New approaches to therapy, such as the use of Eltrombopag in adult patients with ITP, have shown their effectiveness. These data are especially important for those patients who need to delay or avoid splenectomy. Thrombopoiesis factors (AMG531, Eltrombopag) can become a breakthrough in the treatment of ITP, including refractory forms of the disease, due to their high efficiency, shown in the course of studies.Currently, one of the effective therapeutic approaches in the treatment of chronic ITP is available for the treatment of adult patients, which is based on the use of thrombopoietin receptor agonists, one of them is eltrombopag (Revolade). It is a tablet preparation that acts like thrombopoietin, the main substance that regulates the production of platelets in the body [1,2]. Eltrombopag differs from thrombopoietin in terms of its effect on platelet aggregation. Unlike thrombopoietin, the effect of eltrombopag on healthy human platelets does not increase aggregation under the action of adenazine diphosphate (ADP) and does not stimulate the expression of P- selectin. Eltrombopag does not prevent platelet aggregation under the influence of ADP or collagen.The purpose of this study was to determine the safety and efficacy of human recombinant thromboietin (rHTP) for the treatment of chronic ITP and the side effects on the body during the use of this drug.Scientific novelty - based on the use of a new drug from among the thrombopoietin receptor agonists for the treatment of ITP, one of them is eltrombopag (Revolade).
2. Materials and Methods
- The study included patients aged 18 to 55 years with signs of hemorrhagic syndrome and no positive response to corticosteroids. Patients with the following conditions or diagnoses were excluded: virus-induced thrombocytopenia, functional disorders of the heart, kidneys, liver or lungs. All patients received Revolade at a dose of 50 mg per day. The treatment was carried out in both inpatient and outpatient settings. The course of treatment was prescribed for several months. We studied the results of the first two weeks, the period that determines the further tactics of managing patients.Hemogram control was carried out on the day of treatment and every seven days. The hemogram was performed on the sysmex apparatus.
3. Results and Discussions
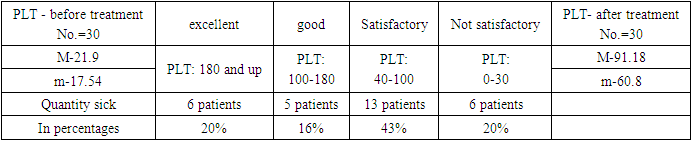
- In RSSPMC, patients with immune thrombocytopenia, with platelet levels from single to 20.10 9 /l were treated with recombinant thrombopoietin - Revolade 50 mg per day by taking a tablet inside. At the same time, for the relief of hemorrhagic syndrome, patients were prescribed hemostatic therapy. All patients underwent glucocorticoid therapy at different times. Prednisolone or methylprednisolone was most often used, the daily dose was 1-1.5 mg/kg of the patient's body weight. The course of treatment lasted 3-6 weeks. Some patients underwent pulse therapy with methylprednisolone. Almost 80% of patients responded to glucocorticoid therapy. But in the future, these patients experienced a rapid decrease in platelets and frequent relapses. In 20% of patients, an increase in the number of platelets was not observed. Two patients from this group underwent splenectomy a few months ago with a temporary improvement in the first months.
|
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML