-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2023; 13(7): 867-871
doi:10.5923/j.ajmms.20231307.03
Received: Jun. 8, 2023; Accepted: Jun. 29, 2023; Published: Jul. 8, 2023

Prostate Microanatomy of Mature Rats and Its Reactive Changes in Chronic Alcoholism
Radjabov A. B.
Bukhara State Medical Institute, Uzbekistan
Correspondence to: Radjabov A. B., Bukhara State Medical Institute, Uzbekistan.
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The article presents the results of a study on the histological relationship of glandular and non-glandular structures of the prostate gland of mature rats and its structural changes in chronic alcoholism.In rats with chronic alcoholism, compared with the control, there is an increase in the number of acini in the field of view due to a decrease in the diameter of their lumen, a decrease in the volume fraction of the glandular parenchyma in the structure of the organ. The experiment revealed acini with foci of epithelial stratification, cell proliferation and desquamation of epithelial cells.Chronic exposure to alcohol leads to a pronounced degree of lymphocytic infiltration with lymphoid nodular formation, to a moderate form of connective tissue proliferation, in the interglandular stroma the number and diameter of vessels increase and their wall thickness decreases.
Keywords: Rat prostate, Gland, Morphometry, Chronic alcoholism
Cite this paper: Radjabov A. B., Prostate Microanatomy of Mature Rats and Its Reactive Changes in Chronic Alcoholism, American Journal of Medicine and Medical Sciences, Vol. 13 No. 7, 2023, pp. 867-871. doi: 10.5923/j.ajmms.20231307.03.
Article Outline
1. Introduction
- The study of structural and functional changes in the prostate gland is one of the topical areas of modern experimental medicine. The morphostructure of the prostate gland can be influenced by various environmental factors, including chemical ones. Alcohol is one of the most toxic xenobiotics, which has a detrimental effect on almost all organ systems [1,3,5,6,7,9,10,11,12,13].According to the World Health Organization, as a result of the harmful use of alcohol in the world, 3 million deaths are recorded every year, which is 5.3% of all deaths [2].It should be noted that in a comparative aspect, the morphology of the prostate at puberty and under chronic alcohol exposure with a description of the morphometry of all glandular and non-glandular structures has not been practically studied in a comprehensive manner. In this regard, the study of the morphological features of an organ under conditions of chronic alcohol exposure is of undoubted interest for theoretical and practical medicine.
2. Purpose of the Study
- To study the histological structure of the epithelial-stromal elements of the prostate of mature rats and rats with chronic alcoholism.
3. Material and Methods
- The study was performed on 26 outbred white male rats at the age of 6 months. 2 experimental groups were formed: 1st - control (n=14); 2nd – experimental group (n=12).In the experimental group, for modeling chronic alcoholism, forced alcoholization of animals using 40.0% ethanol solution was used [8]. The solution was administered intragastrically using a metal probe 1 time per day at a total dose of 7 g/kg of body weight for 1 month before the study age. Control animals received intragastrically equal volumes of 0.9% NaCl solution. Rats were killed by instantaneous decapitation under ether anesthesia, according to approved rules [4].For histological examination, pieces of the prostate were fixed in 10% buffered formalin and embedded in paraffin according to the standard method. Histological sections obtained from paraffin blocks, 5-7 μm thick, were stained with hematoxylin and eosin for review purposes, collagen fibers were detected by van Gieson staining.With a microscope magnification of 70 times (7x10), the sections were determined:- the shape of the lumen of the glands, the number of terminal sections of the glands in the field of view, the volume fraction of acini with and without secretion (in %), the number of acini with desquamated epithelial cells in the field of view, in the intralobular stroma, the number of intraorgan vessels in the field of view was counted;In the preparations, at a magnification of 280 times (7x40), using an eyepiece micrometer, the diameter of the lumen of the glands, the height of the epithelium, the inner diameter and wall thickness of intraorganic vessels were measured. In addition, the thickness of collagen fibers and their distribution in the tissues of the gland were determined.In the field of view (7x40), the presence and severity of lymphocytic infiltration in the tissues of the gland was assessed. When distributing lymphocytes by severity (cell density), the classification of the North American Chronic Prostatitis Collaborative Research Network and the International Prostatitis Collaborative Network was used:1) mild degree - single lymphocytic cells separated by distinct intermediate zones;2) moderate degree - confluent fields of lymphocytic cells without tissue destruction and / or lymphoid nodular / follicular formation;3) severe degree - confluent fields of lymphocytic cells with tissue destruction and / or lymphoid nodular / follicular formation.To assess the severity (fibrosis) of the proliferation of connective tissue using an eyepiece micrometer with a magnification of the objective x40, eyepiece x7 in the field of view, the thickness of the stroma layers between the glands was measured.The degree of compaction of the connective tissue was determined by the appropriate method (Gorbunova E.N., Davydova D.A., Krupin V.N., 2011) as follows: mild form (increase in the thickness of stromal septa up to 2 times in 2-4 fields of view out of 10); 2) moderate form (the thickness of the stromal septa is increased up to 2 times in more than 4 fields of view or a sharp thickening - more than 3 times and is present in single (1-2) fields of view); 3) pronounced form (stromal septa are enlarged up to 3 times or more in 7-10 fields of view).A study was made of the volume fractions of glandular and stromal elements (in %). To do this, using the morphometric grid G.G. Avtandilov (with the number of intersections 100) using an eyepiece x10, a lens x10 in each preparation of the prostate in 10 fields of view, the number of intersections falling on the stromal and glandular (including the lumen of the gland) elements was counted to determine their ratios.
4. Rеsults аnd Disсussiоn
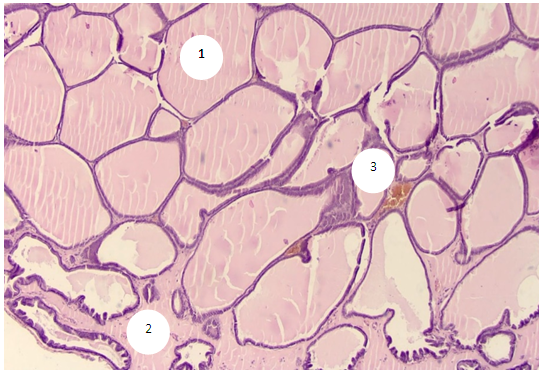
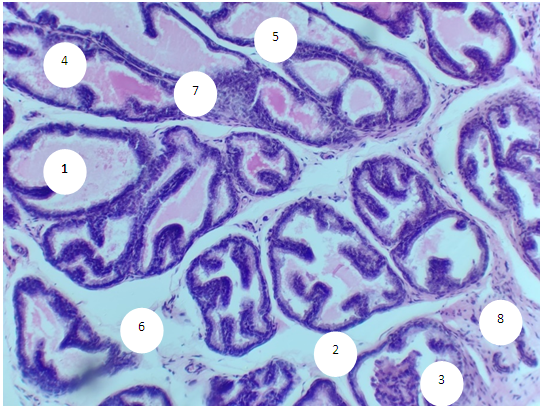
- The study showed that in 6-month-old rats, the prostate has a normal structural plan, consists of numerous separate alveolar-tubular glands and muscular-elastic stroma in the form of loose fibrous connective tissue, bundles of smooth myocytes and vessels.Plain microscopy reveals single epithelial-stromal outgrowths in the acini, the terminal sections are represented by highly prismatic epithelium with high columnar and basal cells resting on a clearly visible basement membrane. The height of the epithelial layer varies from 12.6 to 21.0 µm, on average, 17.8 ± 0.38 µm. Acini have oval and rounded shapes (Fig. 1). The diameter of the lumen of the glands ranges from 210.0 to 441.0 microns, on average - 330.5 ± 9.7 microns. The number of acini in the field of view ranges from 18 to 28, averaging 22.0±0.5. The volume fraction of acini with a secret is in the range of 90.0-100%, on average - 93.3±0.5. The proportion of acini without a secret is 0-10.0%, on average 6.7±0.5. Acini with desquamated epithelial cells were not found in the preparations.In the periglandular stroma, single scattered lymphocytes are determined, separated by clear intervals. Their number in the field of view ranges from 8 to 12, on average 10.0±0.22. In the preparations, a thin stroma is determined, in most cases the acini are located back to back. The thickness of the stromal septa between the acini ranges from 12.6 to 33.6 µm, averaging 23.1±1.13 µm.The number of stromal vessels in the field of view is in the range of 4-9, averaging 7.0±0.3. The inner diameter of the venules is in the range from 21.0 to 29.4 microns, on average - 24.8±0.42 microns. The thickness of their wall ranges from 4.2 to 8.4 microns, on average - 5.8 ± 0.21 microns. The diameter of the capillaries varies from 8.4 to 16.8 µm, on average 13.1±0.42 µm. The wall thickness is in the range of 2.1-4.2 microns, on average - 4.12±0.13 microns. The inner diameter of arterioles ranges from 12.6 to 16.8 µm, averaging 14.7±0.21 µm. Their wall thickness varies from 4.2 to 8.4 µm, on average 7.69±0.21 µm.
5. Conclusions
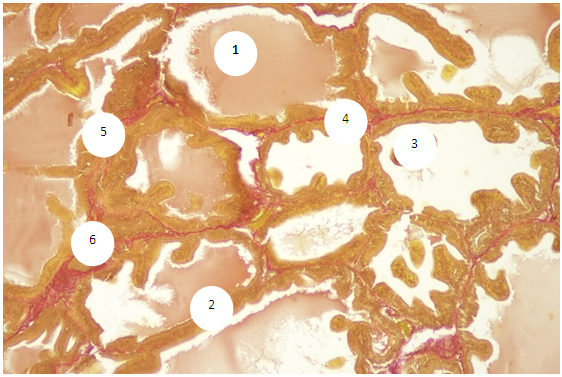
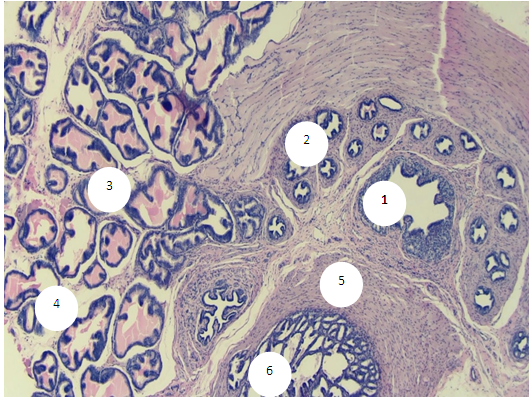
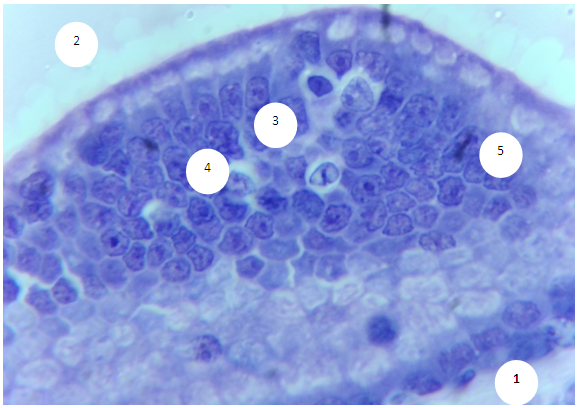
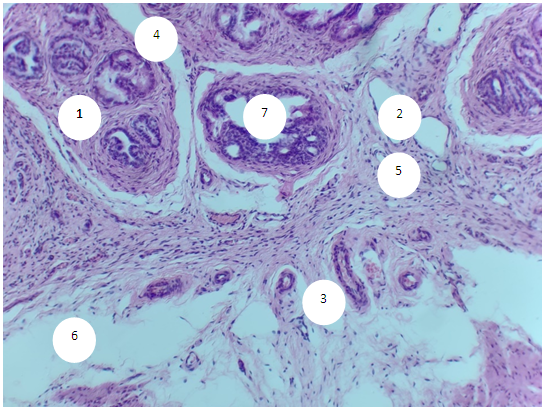
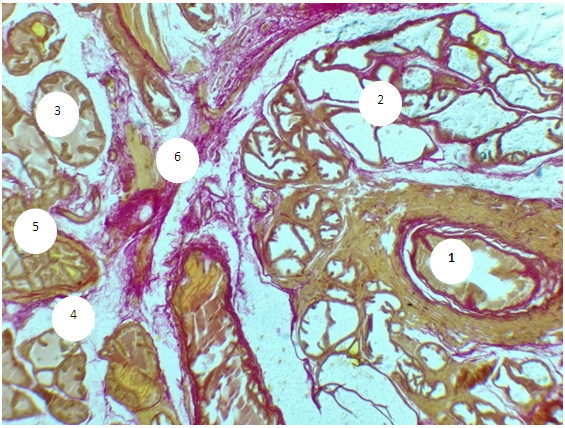
- The prostate of 6-month-old rats shows morphological features typical of an organ with complete differentiation of the development of glandular-stromal elements.In animals with chronic alcoholism, polygonal glands are observed, an increase in the number of acini in the field of view due to a decrease in their diameter, a decrease in the volume fraction of the glandular parenchyma in the structure of the organ, alterative changes in the form of focal desquamation of varying severity, which indicates an accelerated elimination of glandular epithelial cells. In places, acini are detected with foci of epithelial stratification, which can subsequently cause a malignant neoplasm.Involutive changes in the form of flattening of the acinar epithelium, a decrease in the size of the acinus and a decrease in secretory activity are combined with the proliferation of acinar epithelial cells, which leads to the formation of cribriform and papillary structures.In the experiment, the number and diameter of vessels increase, their wall thickness decreases, pronounced diffuse-focal periacinous lymphocytic infiltration of stromal interlobar tissue with lymphoid nodular formation is noted, in some areas periacinar lymphoid infiltration, destroying the basal plate, extends to the epithelial layer.Alcohol exposure leads to a moderate form of expansion of connective tissue layers, especially in the subcapsular zone, pathological growth of fibrous connective tissue and compaction of bundles of collagen fibers, which can be considered as a reaction of the body aimed at isolating the lesion from surrounding tissues and systemic blood flow. Ultimately, the connective tissue replaces the parenchymal elements, which leads to a decrease, and sometimes to the loss of the functions of an organ or tissue.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML