Goyibova Nargiza Salimovna, Garifulina Lilya Maratovna
Department of Pediatrics, Faculty of Medicine, Samarkand State Medical University, Uzbekistan
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
51 children were examined with exogenous constitutional obesity with the presence of abdominal obesity in 24 children. The studies were carried out based on family polyclinics in Samarkand. A laboratory examination of children with the determination of partial kidney functions was carried out. It was found that the glomerular filtration rate (GFR) depended on the type and severity of obesity, with an increase in the degree of obesity in children, an increase in GFR was noted, and in children with abdominal obesity, hyperfiltration was observed more frequently with significantly higher rates compared to children with simple obesity. GFR did not depend on the duration of obesity, but depended on the level of blood pressure, especially in the group with abdominal obesity. In children with abdominal obesity, there was a high frequency of children with microalbuminuria, which characterized a violation of the glomerular-tubular balance in the kidney, and in 5.6% of children, there was a violation of the concentration function of the kidneys, which required specialized assistance from a pediatric nephrologist.
Keywords:
Obesity, Children, Glomerular filtration rate, Microalbuminuria
Cite this paper: Goyibova Nargiza Salimovna, Garifulina Lilya Maratovna, The State of Partial Kidney Functions in Children with Obesity, American Journal of Medicine and Medical Sciences, Vol. 13 No. 7, 2023, pp. 863-866. doi: 10.5923/j.ajmms.20231307.02.
1. Introduction
To date, the problem of obesity, including in children, continues to be one of the main health problems in the world. The International Obesity Task Force has estimated that 22 million children under 5 years of age are overweight or obese [1]. Also between the ages of 5 and 17, about 1 in 10 children are overweight, which is about 155 million. Of these, 45 million suffer from obesity of varying severity. At the same time, in recent years, abdominal obesity has spread with great frequency, which, being a component of the metabolic syndrome, has several complications that have manifestations both in childhood and in subsequent adulthood [2,3].Scientific studies in recent years indicate that abdominal obesity is accompanied by kidney damage. In their works, J. Chen et al. [4,5] showed that abdominal obesity with complications is an independent risk factor for chronic kidney disease. The presence of abdominal obesity increases the likelihood of developing CKD in patients older than 20 years by 2.6 times, and it increases with the increase in the number of complications of abdominal obesity.The pathological influence of obesity is also observed in childhood. Taking into account that the impact of obesity on the cardiovascular system is observed in children and adolescents with low insulin sensitivity, we can assume the development of complications of obesity, including the impact on the kidneys even in childhood [6,7]. In the presence of childhood obesity, the kidneys are unable to cope with the physiological stress that arises from rapid body growth and leads to metabolic dysfunction later in life [7].One of the early disorders of the functional state of the kidneys may be microalbuminuria, changes in the kidney filtration rate (GFR), and other partial kidney functions. In connection with the above, the purpose of our study was to study the partial functions of the kidneys in obese children.
2. Material and Methods
Our studies were carried out in family clinics in the city of Samarkand (Uzbekistan). The main group consisted of 51 children with exogenous-constitutional, alimentary obesity and 20 children of the control group with normal body weight.Anthropometric studies were carried out using standard measuring instruments (floor stadiometer and medical scales). Anthropometric measurements included: height, body weight, waist, and hip circumference. A comparison of the obtained data and assessment of physical development was carried out according to the WHO centile tables of age and gender distribution of height and body weight for children aged 5-19 years. Body mass index (BMI) was calculated based on the measurements. The results were evaluated using standard deviations of BMI (SDS) and Z- score following WHO recommendations [8]. Waist circumference (WC) was measured at the midpoint between the iliac crest and costal arch in the midaxillary line in a standing position at the end of full exhalation, the result was compared with the percentile distribution of waist circumference (cm) in boys and girls aged 2 to 18 years (VNOK 2009) [9].Following the tasks set, the children of the main group were divided into 2 groups: group 1 - 27 children had a BMI +2.0 to ≥+3 SDS, i.e. children had a BMI characterizing obesity from 1 to 3 degrees, but with WC being within the normal range according to percentile deviations, respectively, for age and sex. The average BMI was 31.92±0.51 kg/ m2.Group 2 - 24 children included children with BMI +2.0 to ≥+3 SDS, which was, respectively, obesity groups 1-3, FROM the waist in this group exceeded the 90th percentile for the corresponding age and gender. The average BMI in children of this group (32.43±0.62 kg/m 2) was significantly higher than in the control group (p < 0.01).The control group consisted of 20 children of the same age (average 12.00±0.34 years) with a normal body weight of 19.18±0.34 kg/m 2 (BMI less than +1 SDS for a given sex and age).The main group of children with simple obesity, abdominal obesity, and the control group were comparable in age (p>0.05) and gender composition (p>0.05).The determination of the glomerular filtration rate was carried out according to the Cockcroft-Gault formula.Microalbuminuria was determined in morning urine using MICRAL - TEST test strips II (Roche Diagnostics) (with a sensitivity limit of 0 to 100 mg/l). A diagnostic positive level of albumin excretion was considered to be 20 mg/l and higher in the morning urine. We conducted a two-time study with a break from a week to 7 days. A positive test was considered if there was a double presence of microalbuminuria in the morning urine. The second stage was the determination of albumin by the immunoturbidimetric method on the apparatus "Integra Analyzer" ("Roche, Basel, CH") in the morning urine. Based on the obtained data (albumin concentration in 1 ml of urine), albumin excretion (mg/l) was calculated. A positive test was considered in the presence of albumin above 20 mg/l in a single portion of morning urine.Statistica 10 program. Methods of variational parametric and nonparametric statistics were used with the determination of the arithmetic mean (M), standard deviation (s), standard error of the mean (m), and relative values (frequency, %). The statistical significance of the measurements obtained was determined by Student's t-test (t) with the calculation of the error probability (P).
3. Research Results
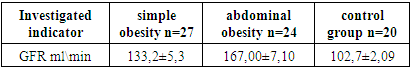
When assessing the filtration function of the kidneys in obese patients, inaccuracies are observed, since the body surface area in obese patients does not correspond to standard indicators, and therefore, when determining by calculation methods, GFR values are underestimated or overestimated. Also in pediatrics, recommendations in this direction are single and do not reflect clear recommendations for monitoring GFR in children with excessive fat deposition. Literature data indicate an increase in GFR with an increase in body mass index, regardless of age, in this regard, we used the Cockcroft-Gault formula taking into account the patient's body weight, while in the Schwartz and Kounahan formulas recommended for use in pediatric practice, only the child's height is used.Table 1. GFR value according to Cockcroft-Gault in comparison groups
 |
| |
|
When calculating GFR using the Cockcroft-Gault formula, a statistically significant increase in filtration rate was obtained from the control group to the group with abdominal obesity (Table 1).In the control, the state of hyperfiltration i.e. GFR above 130 ml/min/1.73 m 2 was not observed in children with normal body weight and averaged 102.7±2.09 ml/min.In children with simple obesity, the frequency of children with a state of hyperfiltration was 6 children (22.2%), while the average rate in children of this group was above the norm and was statistically significant compared to the control (133.2 ± 5.3 ml/min; p < 0.01), no cases of children with a hyperfiltration state were observed.In children with abdominal obesity, the frequency of increased glomerular filtration rate was 19 children (70.3%), with an average level of 167.00±7.10 ml/min, which was significantly higher compared to the control (p < 0.01), it should be noted that in 2 children (7.4%) from the group with visceral obesity, a state of hyperfiltration was observed in 60 to 70 ml/min on average 6 7.85 ± 5.00, which was regarded as the onset of chronic kidney disease in children: kidney damage with a slight decrease in GFR. This fact contributed to the taking control of this contingent of children by a pediatric nephrologist.We were interested in studying the state of GFR depending on the BMI and the duration of obesity in children.The obtained data showed that the glomerular filtration rate changed depending on the BMI. The state of hyperfiltration was observed in children with grade 3 obesity, with ≥+3 SDS: 173.00±5.58 ml/min, while the indicators significantly differed from those of children with grade 1-2 obesity (BMI>+2<+3 SDS) 155.77±5.00 ml/min (p<0.01), overweight (+1.0 to +2.0 SDS) - 146.10±6.33 ml/min (p<0.01) and control <+1 SDS 102.7±2.09 ml/min (p<0.01).When carrying out a correspondence with the duration of the course of obesity in children, it was revealed that the state of hyperfiltration did not always occur in children with a long course of the pathological process in the form of obesity. The data obtained showed that the level of GFR did not differ significantly from each other in the groups of children depending on the duration of obesity. So, with a duration of obesity of 2-3 years, the average level of GFR was the lowest (149.23 ± 4.86 ml/min), and with a duration of obesity of 7 years or more, the highest values (162.33 ± 7.21 ml/min), with an average level of 153.12±6.45 ml/min for 4-6 years, but these differences did not differ statistically significantly (p > 0.05). Given the statistical data, it can be said that GFR does not depend on the duration of obesity, but depends on body weight since patients can gain quite a high body weight in a short period of obesity.When carrying out the correspondence with the level of blood pressure in children, the dependence of the level of GFR on the percentile distribution of blood pressure depending on age and height, and sex was revealed, the distribution of indicators of which was recommended in the Clinical guidelines of the VNOK (2009) [9].The data obtained showed that despite the higher level of GFR in the group of children with simple obesity with high normal blood pressure (18 children - 132.12±5.8 ml/min), it did not have a significant difference with the group of children with normal blood pressure. (10 children - 110.15±6.3 ml/min), i.e. BP level did not affect GFR in the group of children with simple exogenous constitutional obesity.Whereas in the group of children with abdominal obesity, there was a significant difference between the glomerular filtration rate depending on the level of blood pressure, with average normal values in children with normal blood pressure (8 children - 113.28 ± 4.93 ml/min), the glomerular filtration rate in children with normal high blood pressure was significantly higher (11 children -156.38 ± 4.93; p<0.001) compared with children with normal blood pressure. Also, significantly higher GFR compared with children with normal BP and high normal BP had children diagnosed with stage I hypertension (≥ 95-<99 percentile) 3 children - 180.5 ± 6.3 ml/min (p<0.01 compared with high normal blood pressure), and children with II degree hypertension (≥ 99th percentile) 2 children - 174.71 ± 4.38 ml/min (p<0.01).Thus, the filtration fraction increases simultaneously with an increase in blood pressure. Changes in renal hemodynamics can occur already at an early stage of hypertension and in the period preceding hypertension. Even in a newborn whose parents both suffer from hypertension and have normal blood pressure, a marked decrease in renal blood flow and an increase in the filtration fraction were found [7,10,11]. This suggests that renal hyperperfusion is a very early sign, and possibly a prerequisite for the development of hypertension.Thus, when assessing the functional state of the kidneys using the determination of GFR according to the Cockcroft-Gault method in the compared groups of patients, it was found that the majority of obese children had hyperfiltration in terms of glomerular filtration. The results obtained are consistent with the data literature that one of the main pathogenetic factors in the development And progression of kidney damage in obesity is intraglomerular hyperfiltration and hypertension [7].Microalbuminuria is considered one of the important markers of kidney damage and the development of chronic kidney diseases (impaired microcirculation in the kidney), especially primary lesions of the glomerular apparatus of the kidneys [12]. Also in our work, we determined the level of albumin excretion in the morning portion of urine, which showed that the frequency of a high level of microalbuminuria increased depending on the type of obesity and its severity.In the study of the level of albumin in the urine in patients with simple obesity: pathological MAU was detected in 6 (22.2%) patients, in 18 (66.6%) patients it was within the physiological range, and in 3 (11.1%) patients there was no. In children with abdominal obesity, pathological MAU was detected in 14 (58.33%) patients (p < 0.01 compared with group 1), and only in 9 (37.50%) patients were within the physiological range (p < 0.05 compared with the first group), there were no cases of absence of MAU. It was found that the average level of microalbuminuria increased depending on the severity of obesity, with an average level of 18.00±4.89 mg/l in children with overweight, it was statistically significantly higher in children with obesity of 1-2 degrees (BMI>+2 <+3 SDS) 37.62 ± 8.14 mg/l (p<0.05) and grade 3 obesity, with ≥+3 SDS 64.30±2.21 mg/l (p<0.0001).The concentration function of the kidneys was studied using the Zimnitsky method. Studies have shown that the average urine density in both study groups was the reference values, 1017.09 ± 2.18 in group 1 and 1016.84 ± 1.57 in the second group (p> 0.05), while the minimum urine density was 1006, and the maximum is 1028.The low specific gravity of urine (hypostenuria) (specific gravity of urine below 1010) in a single portion of urine was observed in 1 (3.7%) child from group 1, while it was combined with low specific gravity in all portions of urine (isosteric). In the group of children with abdominal obesity, more than 4 cases (16.6%) cases of hyposternuria were observed, while in 2 (8.33%) cases it was accompanied by isosthenuria and nocturia (prevalence of nocturnal diuresis), which indicated damage to the tubular apparatus of the kidneys and indicated a violation of the processes of concentration in the renal tubules. In other cases, the density of urine during the day was within the normal range, i.e. the concentration function of the kidneys was preserved.Diuresis per day was 1.47 ± 0.05 l / day in group 1 and 1.5.0 ± 0.04 l / day in group 2 (p> 0.05), daytime diuresis prevailed over nighttime and amounted to 0, respectively. 90 ± 0.06 l in the 1st group and 0.91 ± 0.07 in the second group.Thus, in almost an absolute number of obese patients (in 48 children out of 53 90.5%), the concentration function of the kidneys was preserved, while in 3 children (5.6%) there was a dysfunction of the distal tubules. At the same time, disorders of the concentration function of the kidneys in patients with obesity depended on the presence of abdominal obesity, which is often accompanied by complications.
4. Conclusions
Thus, GFR depended on the type and severity of obesity, with an increase in the degree of obesity in children, an increase in glomerular filtration rate was noted, and in children with abdominal obesity, hyperfiltration was observed more frequently, while the average values were significantly higher compared to children with simple obesity.GFR did not depend on the duration of obesity, but depended on the level of blood pressure, especially in the group with abdominal obesity.In the group of children with abdominal obesity, there was a high frequency of children with microalbuminuria, the average level of which had significant differences from children with simple obesity, which characterizes abdominal obesity as a condition in which there is a loss of glomerular tubular balance.Abdominal obesity in 5.6% of cases was accompanied by disruption of the distal tubules with damage to the concentration function of the kidneys, which required specialized assistance from a pediatric nephrologist.
References
| [1] | World Health Organization. Report on the fifth round of data collection, 2018–2020: WHO European Childhood Obesity Surveillance Initiative (COSI). 2022. 70 r. |
| [2] | Ruiz Lyndsey D., Zuelch Michelle L., Dimitratos Sarah M., Scherr Rachel E.. Adolescent Obesity: Diet Quality, Psychosocial Health, and Cardiometabolic Risk Factors. Nutrients. 2019 Dec; 12(1): 43. doi: 10.3390/nu12010043. |
| [3] | Peterkova AV, Bezlepkina OB, Vasyukova OV, i dr. Ozhirenie u detei. Clinics recommendations. - M.: Ministry zdravookhraneniya Rossiiskoi Federatsii; 2021. |
| [4] | Chen J, Muntner P, Hamm LL, et al. The metabolic syndrome and chronic kidney disease in US adults. Ann Intern Med. 2004 Feb 3; 140(3): 167-74. PMID: 14757614. |
| [5] | Melnik A.A. Metabolic syndrome and the risk of chronic kidney disease. / / Kidneys - Volume 6, No. 2, 2017 -С 80-90. |
| [6] | E.K. Petrosyan et al. Functional state of the kidneys in adolescents with obesity // Nephrology. 2017. Volume 21. No. 2 - P.48-55. |
| [7] | Vyalkova AA, Lebedeva EN, Krasikov SI et al. Clinical and pathogenetic aspects of kidney damage in obesity (literature review). Nephrology 2014; (3): 24-33 [Vyalkova AA, Lebedeva EN, Krasikov CI i dr. Clinical pathogenetics aspect povrezdenia pochek pri ogirenii. Nephrologia 2014; (3): 24-33]. |
| [8] | World Health Organization. Obesity and overweight. Factsheet No. 311. January 2015. Online resource: http://www.who.int/mediacentre/factsheets/fs311/ru. |
| [9] | National clinical guidelines of the All-Russian Scientific Society of Cardiology. Moscow. - 2009. S. 392. |
| [10] | Wang Y, Chen X, Song Y et al. Association between obesity and kidney disease: a systematic review and metaanalysis. Kidney Int 2008; 73: 19-33. |
| [11] | Sarafidis PA, Ruilope LM. Insulin resistance, hyperinsulinemia, and renal injury: mechanisms and implications. Am J Nephrol 2006; 26: 232-244. |
| [12] | Gaysina L.R., Safina A.I., Valeeva F.V. The state of lipid metabolism and its relationship with microalbuminuria in children and adolescents with obesity // Siberian Medical Journal, 2011, Volume 26, No. 4, Issue 2 - C 157-160. |


 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML