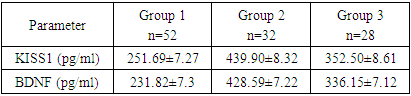-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2023; 13(6): 849-855
doi:10.5923/j.ajmms.20231306.15
Received: May 28, 2023; Accepted: Jun. 20, 2023; Published: Jun. 27, 2023

Premature Ovarian Insufficiency Related to COVID-19: Role of Hypothalamic Markers
Khaydarova Feruza Alimovna, Bakoeva Nilufar Matyokub Qizi
Republican Specialized Scientific and Practical Medical Center of Endocrinology named after Y. Kh. Turakulov, Tashkent, Uzbekistan
Correspondence to: Bakoeva Nilufar Matyokub Qizi, Republican Specialized Scientific and Practical Medical Center of Endocrinology named after Y. Kh. Turakulov, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Premature ovarian insufficiency (POI) is the cessation of ovarian function before the age of 40 years. Background. Being the main cause of infertility and a decrease in the quality of life of women, POI is one of the most difficult problems of female reproduction. After a confirmed diagnosis, there are currently no methods to restore ovarian function and fertility. Therefore, it is important to identify risk factors for the development of POI at an earlier stage. Purpose of the study isdetermination of hypothalamic markers such as kisspeptin (KISS1) and brain-derived neurotrophic factor (BDNF) in the development of premature failure in women after COVID-19. Materials and methods. Data of clinical and laboratory examination of 112 women: 52 women with POI who developed after COVID-19, 28 women with established POI without COVID-19, 32 women of childbearing age with a regular menstrual cycle. Results. The most pronounced decrease in the concentration of kisspeptin (less than 298.1 pg/ml) was observed in patients with POI after suffering COVID-19, and in healthy women, higher levels of kisspeptin (more than 401.16 pg/ml) were found. Of interest was the fact that in women with POF in the comparison group, kisspeptin levels (ranging from 310 to 387.1 pg/ml) were slightly lower than in healthy women. The average blood BDNF was significantly lower in women of the main group (231.82±7.3 pg/ml) compared to the control group (428.59±7.22 pg/ml). In women with POI in the comparison group, the level of brain-derived neurotrophic factor was lower (336.15±7.12 pg/ml) than in healthy women. Based on this, we can conclude that the level of BDNF in women with premature ovarian failure is lower than in healthy women, in the case of a coronavirus infection, its level decreases even more. Conclusions. Kisspeptin can be considered as a promising neuropeptide for the regulation of the female reproductive system and for the correction of reproductive disorders. Brain-derived neurotrophic factor should be considered as a marker of depression.
Keywords: Premature ovarian insufficiency, Kisspeptin, BDNF, COVID-19
Cite this paper: Khaydarova Feruza Alimovna, Bakoeva Nilufar Matyokub Qizi, Premature Ovarian Insufficiency Related to COVID-19: Role of Hypothalamic Markers, American Journal of Medicine and Medical Sciences, Vol. 13 No. 6, 2023, pp. 849-855. doi: 10.5923/j.ajmms.20231306.15.
1. Introduction
- Today, premature ovarian insufficiency (POI) is one of the most difficult problems of women's health. One of the first medical descriptions of POI in a girl at the age of 17 was published in 1920 by the doctor R.A.Kisch. In 1925, the psychoanalyst H.Deutsch described a 35-year-old woman with premature menopause. All researchers describing cases of premature stop of ovarian function in the first half of the 20th century noted the extreme rarity of this pathology. According to the World Health Organization (WHO), "... the incidence of POI ranges from 1-3% to 10% of the female population". Premature ovarian failure (POF) is the cessation of ovarian function before the age of 40 years. This is due to hypoestrogenism and loss of residual follicles, leading to menstrual irregularities, infertility and reduced health-related quality of life. The European Society for Human Reproduction and Embryology (ESHRE) suggests the following diagnostic criteria: amenorrhea or oligomenorrhea for at least four months and elevated follicle-stimulating hormone (FSH) > 25 IU/L [1]. According to epidemiological studies, this disease is closely related to age: in women under the age of 20, POI occurs with a frequency of 1:10,000, and between the ages of 30 and 40 - 1:1,000 [2]. According to the research results of Khaydarova F.A., Fakhrutdinova S.S. (2019), the incidence of POI in Uzbekistan is 2.5%. The average age of women with POI is 31.4±0.5 years [3].The coronavirus itself, as well as the measures taken to reduce its spread, have seriously affected the lives of the world's population. The pandemic has significantly affected the mental health of many people in the population, leading to loneliness, social isolation, financial stress, as well as anxiety and fear of contracting the virus and uncertainty about the future. It is known that periods of stress and psychological distress can affect women's reproductive health. Stressors can affect the hypothalamic-pituitary- gonadal (HPG) axis and can alter the neuromodulator cascade that governs the regulation of gonadotropin- releasing hormone (GnRH) [4]. It has been proven that dysmenorrhea is associated with high levels of stress and emotional instability [5]. There are more and more questions are being raised regarding the female reproductive system, especially fertility issues, and clarification is required regarding the possible link between COVID-19 and women's reproductive health. Niamh Phelan et al. conducted a survey of women population of reproductive age about their menstrual cycle, libido and changes in their lifestyle during the pandemic. 441 (46%) women who had regular menstrual cycles reported changes in their menstrual cycle during the COVID-19 pandemic. 158 women (17%) did not have a period during the pandemic, which is 4% more than before the pandemic. Women reported a significant increase in suffering from mental health symptoms. Stress has an inhibitory effect on the hypothalamic-pituitary-gonadal (HPG) axis. In response to stress, GnRH release from the hypothalamus is inhibited, and glucocorticoids inhibit the release of luteinizing hormone (LH) and the production of estrogen and progesterone by the ovaries [6,7]. Stress regulates the HPG axis through activation of the hypothalamic sympathetic neural pathways, resulting in the release of norepinephrine in the ovaries. [8]. Severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2) infection can affect the female reproductive system, since oocytes and ovarian tissue express medium-high levels of the Angiotensin-converting enzyme 2 (ACE2) receptor [9]. There was no significant difference in the level of expression of ACE2/ transmembrane serine protease 2 (TMPRSS2) between the ovaries in different ages of women, as well as low and high ovarian reserve [10]. ACE2 plays a key role in the ovaries: it promotes steroid secretion [11], helps follicle development [12] and oocyte growth [13], affects ovulation [14]. As the actual pandemic caused by coronavirus disease (COVID-19) is far from over, we see the importance of talking about the impact on reproductive health.The largest study of over 18,000 mobile application users also found that almost half of the participants reported stress during the COVID-19 pandemic [15]. Interestingly, while a number of participants had more anovulatory cycles (7.7%) or cycles of abnormal length (19.5%) during the pandemic, a number of women actually had fewer anovulatory cycles (9.6%) or cycles of abnormal length (19.6%). The authors suggest that this may reflect that the COVID-19 pandemic likely affected women differently with different sociodemographic characteristics. For example, application users in the study tended to be from high-income countries (US and UK) and had a high level of education. Therefore, some of these women may started working from home instead of commuting to work. Studies have shown that women who started working from home rather than commuting may have more opportunities to exercise or eat healthy, given reduced travel time [16]. Overall, these studies show that there is an association between anxiety, caused by the COVID-19 pandemic and an increased prevalence of menstrual irregularities in women. However, they also emphasize that the response to the COVID-19 pandemic has not affected all women equally. There is an association between anxiety caused by the COVID-19 pandemic and an increased prevalence of menstrual irregularities in women. In addition, another mechanism for the development of POI is stress and its consequences during a pandemic. While it is well known that increased psychosocial stress can lead to menstrual irregularities, this is one of the first studies to assess menstrual irregularities in the context of the COVID-19 pandemic and the first to link such changes to perceived stress. But exactly what mechanism develops a violation of the reproductive system after COVID-19 remains unclear. In recent years, attention has been paid to the role of kisspeptin and BDNF.The discovery of hypothalamic kisspeptin neurons had a profound impact on the world of reproductive neuroendocrinology. Kisspeptin is a neuropeptide of critical importance in the regulation of reproductive function, and dysfunction of kisspeptin neurons leads to reproductive dysregulation. Kisspeptin is a peptide encoded by the KISS1 gene and was first identified in 1996 [17]. The first studies of kisspeptin were carried out in the field of oncology, where the KISS1 gene was first discovered, which is expressed in malignant melanoma cells. Kisspeptin was originally called metastin, because it was first discovered as an inhibitor of metastasis in melanoma cells [18]. The importance of the KISS1/KISS1R system for reproduction was discovered in late 2003, when KISS1R mutations were first discovered in humans and mice suffering from hypogonadotropic hypogonadism. [19]. Kisspeptin soon found itself at the upper level of the reproductive axis. Specialized hypothalamic centers (kisspeptin neurons) have the ability to secrete kisspeptin. Kisspeptin neurons are found in the infundibular nucleus and preoptic region of the human brain [20]. Kisspeptin proved to be one of the strongest stimulators of gonadotropin-releasing hormone (GnRH) secretion in various mammals, including humans [21]. In the study Karakus B. et al. (2019), where the serum level of kisspeptin was lower in women with POI in comparison with healthy women [22]. In the study of Rawa Auda Hussein et al. (2021) showed that the level of kisspeptin in the blood was significantly lower than in women during natural menopause and healthy women [23]. In a study by Gaytan F. et al. in mice with haplo-deficiency of the kisspeptin receptor showed that it causes the development of POI and that this is not associated with a defect in gonadotropin secretion [24]. Long-term persistence of excessive secretion of glucocorticoids leads to glutamatergic hyperactivity, reduction of brain derived neurotrophic factor (BDNF), and a decrease in hippocampal volume. According to a study by Lorkiewicz et al. ACE2 has been shown to be associated with a decrease in BDNF levels [25]. It is widely believed that SARS-CoV-2, by using ACE2 to enter cells, causes its suppression. This mechanism may cause a secondary decrease in BDNF levels. Support for this theory may be reflected in one study that tested patients with COVID-19 for serum BDNF levels. The association of growth factors, especially BDNF, is often reported in the literature, and the likely mechanism by which SARS-CoV-2 may reduce it may prove to be a good biomarker for post-COVID depression [25]. In some studies have shown, that BDNF expression disorders may be associated with the development of POI. BDNF is present in the follicular fluid, where it stimulates the maturation of oocytes into preimplantation embryos [26]. LH release via the paracrine pathway stimulates follicular production of BDNF in granulosa cells, including the Neurotrophic tyrosine receptor kinase 2 (NTKR2) receptor. This mechanism, together with the stimulation of the KISS1R receptor by kisspeptin, ensures oocyte survival and further development [27]. Dorfman et al. also showed that in mice with NTRK2 or KISS1 receptor removed, oocyte decay occurred and, as a result, oocyte cell death. This process induced the POI phenotype in mice. There is also a positive correlation between the number of mature oocytes and the concentration of follicular fluid BDNF [28]. Gaytan et al. [24], causing abnormal signaling between kisspeptin and the BDNF signaling pathway, caused a progressive loss of all classes of follicles in the ovary, leading to premature menopause. The plasma concentration of BDNF has already been studied in women. Begliuomini et al. studied plasma levels of BDNF in a group of patients after natural menopause and in patients with amenorrhea caused by various etiologies, compared with women with a regular menstrual cycle. In both study groups, BDNF concentrations decreased significantly, reaching the lowest level in patients with amenorrhea. In fact, women with regular ovulatory cycles have higher levels of BDNF compared to women with amenorrhea or postmenopause [29]. Czyzyk et al. showed, that plasma concentrations of BDNF in the POI group are significantly lower compared to healthy controls in the late follicular phase. Moreover, the levels of BDNF in the study group were in a very wide range, which revealed a negative correlation between the time interval since the last menstruation and the concentration of BDNF.Purpose of the study is determination of hypothalamic markers such as kisspeptin (KISS1) and brain-derived neurotrophic factor (BDNF) in the development of premature failure in women after COVID-19.
2. Materials and Methods
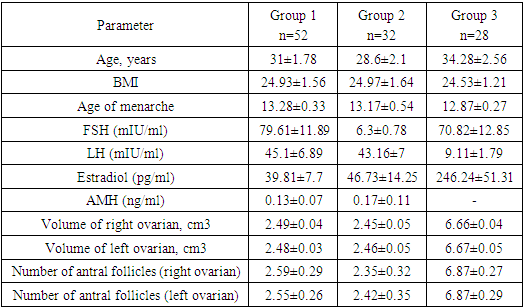
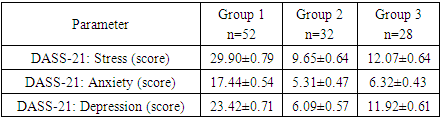
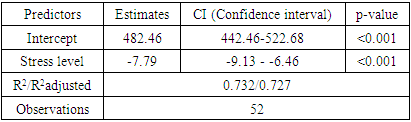
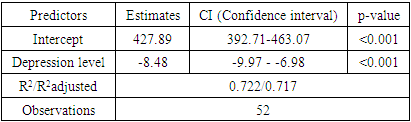
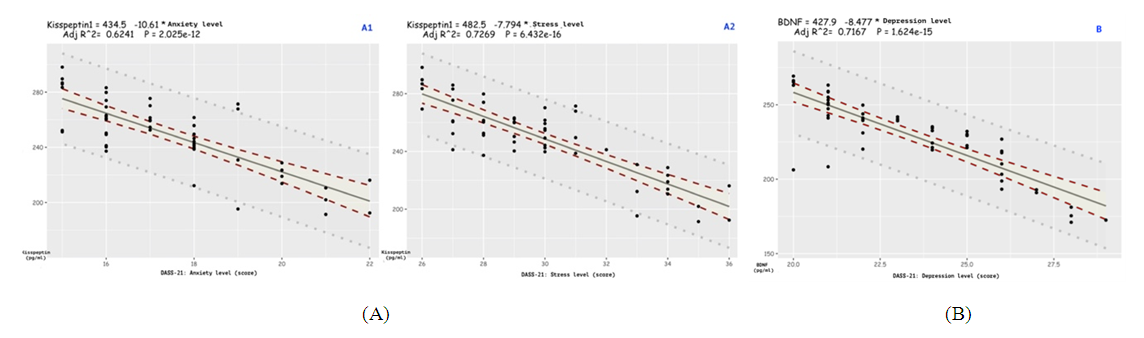
- To achieve this aim, on the basis of the Republican Specialized Medical Center for Endocrinology named after acad. Y.Kh. Turakulova in the department of the "Consultative Polyclinic" in the period from January 2021 to July 2022 examined 112 women. 3 groups of patients were identified: Group 1 - 52 women (mean age 31.05 ± 1.78 years), who had amenorrhea associated with COVID-19, group 2 - 28 women (mean age 34.28±2.56 years) with confirmed diagnosis of POI and group 3 - 32 healthy women (mean age 28.68±2.1 years, with a regular menstrual cycle).Hormonal examination included the determination of luteinizing hormone (LH), follicle-stimulating hormone (FSH), estradiol, anti-Müllerian hormone (AMH) in the blood in women with amenorrhea was determined on any day, and in women with a regular menstrual cycle on days 3-5. Determination of follicle-stimulating hormone (FSH), luteinizing hormone (LH), estradiol (E2), anti-Mullerian hormone (AMH) in blood serum was determined on an automatic immunochemical analyzer "Cobas e411" (Roche Diagnostics GmbH, Germany). The level of kisspeptin (KISS1) was studied using the Human KISS1(Kisspeptin 1) ELISA Kit (Elabscience Biotechnology Co., Ltd, China) and Human BDNF (Brain Derived Neurotrophic Factor) ELISA Kit (Elabscience Biotechnology Co., Ltd, China) for immunosorbent assay with enzyme label (ELISA), based on the principle of "sandwich" on the automatic immunochemical analyzer "Cobas e411" (Roche Diagnostics GmbH, Germany). Ultrasound examination of the pelvic organs was performed using a transvaginal convex probe with a frequency of 7.5 MHz and an abdominal probe with a frequency of 3.5 MHz on a 2000 Toshiba SSA-240 device (Japan). The psychological state of women was determined by the DASS-21 questionnaire, which includes the level of anxiety, stress and depression. Statistical Analysis.Statistical processing of all obtained data was carried out using the Microsoft Excel, Minitab 14 (USA) and RStudio (USA) statistical software packages. The initial data were evaluated for compliance with the normal distribution according to Kolmogorov-Smirnov criterion. Dependences were analyzed using Spearman's rank correlation coefficients. Correlation regression analysis performed according to the Gauss-Markov conditions. To assess the prognostic significance of markers was used a linear regression model. Results are presented as median (Me). Differences were considered statistically significant at р<0,05.
3. Result and Discussion
- In accordance with the tasks set, an analysis of anamnestic data, the results of clinical and laboratory-instrumental examination of 112 women were carried out, divided into 3 groups. There were no significant differences in the mean age of menarche and body mass index in three groups. Menarche up to 12 years was in 2 (1.7%) women, from 12 to 14 years - in 99 (88.3%) women, menarche over the age of 14 years - in 11 (9.8%) women. The functional state of the hypothalamic-pituitary-ovarian system was determined by the results of hormonal examination and transvaginal echography of the pelvic organs. In patients with POI (group 1 and group 2), the levels of FSH and LH in the blood were significantly higher than in women of group 2, and the level of AMH was low in all women with POI. In women with POI (group 1 and group 2), the average ovarian volume did not exceed 3 cm3 and the number of antral follicles in the ovary did not exceed 5 (table 1).
|
|
|
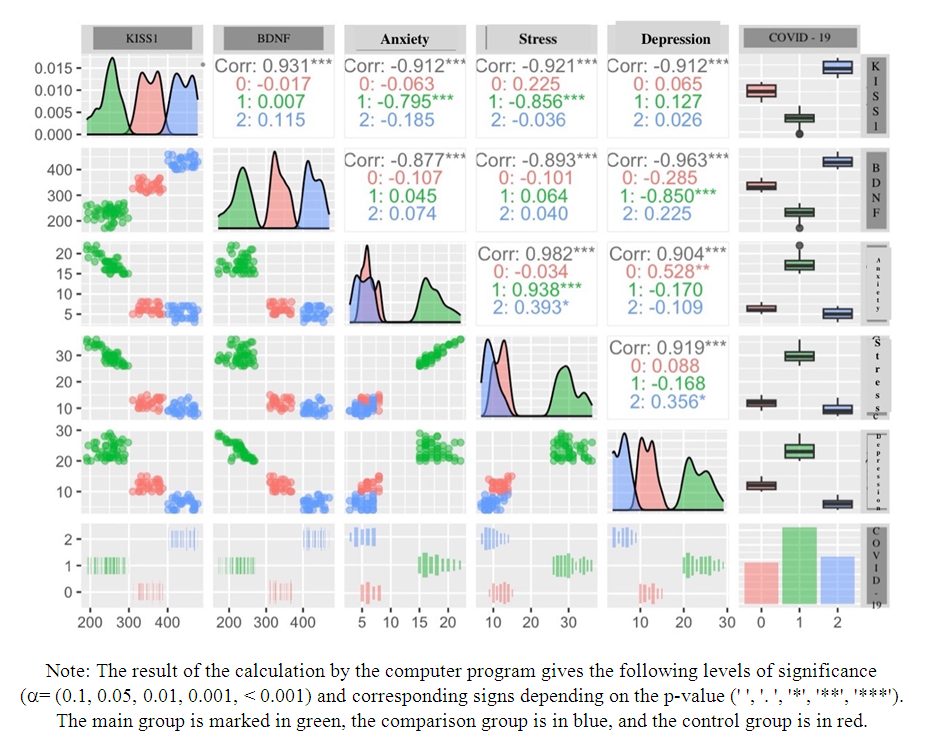 | Figure 1. Paired correlation analysis of indicators KISS1, BDNF, stress, anxiety, depression, and also depending on study groups |
|
|
|
4. Conclusions
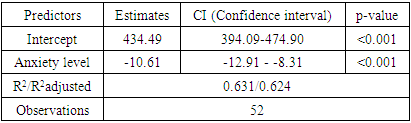
- 1. A questionnaire “DASS-21” for assessing the psycho-emotional state of women after suffering COVID-19 should be used to identify risk groups for developing POI. 2. Anxiety, stress, depression due to COVID-19 in relationship with serum kisspeptin and brain-derived neurotrophic factor are the most significant prognostic indicators in the development of POI. 3. Kisspeptin can be considered as a promising neuropeptide for the regulation of the female reproductive system and for the correction of reproductive disorders. Brain-derived neurotrophic factor should be considered as a marker of depression.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML