-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2023; 13(6): 831-837
doi:10.5923/j.ajmms.20231306.12
Received: Jun. 4, 2023; Accepted: Jun. 22, 2023; Published: Jun. 26, 2023

Obstructive Sleep Apnea: Role of a Dentist
Arifa Bakerywala1, Ashish R. Agarwal2
1Pediatric Dentistry, Tufts University School of Dental Medicine, Boston, USA
2General Dentist in Private Practice, Boston, USA
Correspondence to: Arifa Bakerywala, Pediatric Dentistry, Tufts University School of Dental Medicine, Boston, USA.
| Email: |  |
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Obstructive sleep apnea (OSA), considered the most severe in the spectrum of sleep-disordered breathing (SDB), the overall global prevalence of OSA is 22.6%. Several children experience OSA, prompting medical and dental professionals to raise awareness about proper screening, diagnosis, and treatment. According to the American Academy of Pediatric Dentistry (AAPD), untreated OSA in school-aged children can affect the overall health of children as well as their quality of life. OSA is now being treated with a variety of surgical and noninvasive approaches. Dentists have essential roles in both screening and referral of children with OSA symptoms. The dentist's role has lately developed to include participation in the care of children with OSA through a multidisciplinary approach. This review summarizes the etiology, epidemiology, and management aspects of OSA, with a particular focus on the role of dentists in OSA identification and management.
Keywords: Obstructive sleep apnea, Pediatric obstructive sleep apnea, Sleep-disordered breathing, Oral appliance therapy
Cite this paper: Arifa Bakerywala, Ashish R. Agarwal, Obstructive Sleep Apnea: Role of a Dentist, American Journal of Medicine and Medical Sciences, Vol. 13 No. 6, 2023, pp. 831-837. doi: 10.5923/j.ajmms.20231306.12.
Article Outline
1. Introduction
- The American Academy of Sleep Medicine defines obstructive Sleep Apnea as Obstructive sleep apnea (OSA), as “a sleep-related breathing disorder that involves a decrease or complete halt in airflow despite an ongoing effort to breathe. It occurs when the muscles relax during sleep, causing soft tissue in the back of the throat to collapse and block the upper airway.” [1] Sleep-disordered breathing suggests a range of abnormalities that incorporates snoring, upper airway resistance syndrome, obstructive hypopnea syndrome, and obstructive sleep apnea (OSA) [2]. The overall global prevalence of OSA is 22.6% with its prevalence in men almost twice as high as in women. [3] Sleep-disordered breathing (SDB) in children is a pressing public health issue due to the high frequency of comorbidities such as neurocognitive impairment, cardiovascular issues, and obesity. [2] Pediatric OSA is a childhood condition in which there are upper airway irregularities leading to complete or partial obstruction of the airway during sleep leading to decreased oxygen saturation or awakening from sleep. [4] This condition can have significant effects on their behavior, neurodevelopment, metabolism, and overall growth and development. [4] Early diagnosis, complete evaluation, and timely intervention are imperative to prevent these long-term effects. Young children are more vulnerable to the effects of SDB, and pediatric OSA symptoms and polysomnographic features differ significantly from those in adults. As a result, pediatric SDB and pediatric OSA evaluation procedures have been improved. [5] No significant difference in gender was not reported for the prevalence of OSA in children except among adolescent boys [6]. An increase in weight may be blamed for the higher prevalence of OSA in adolescent boys. [7] Another study proposed that a rise in body mass index (BMI) by 1 kg/m2 as compared to the average raises the risk of developing OSA in children by 12%. [8]. Children who have been breastfed for at least a month or more presented a reduced risk of observed sleep apnea compared to children who never breastfed or breastfed for less than a month. [9] In the pediatric population adenotonsillar hypertrophy is the most common risk factor for SDB followed by obesity, and other craniofacial features such as choanal atresia, micrognathia, narrow arched palate, dolichofacial growth pattern, macroglossia, and retrognathia. [10] [11] [12] In pediatric patients the leading cause of pediatric OSA is adenotonsillar hypertrophy and despite a vast number of healthy children exhibiting the condition, only a few develop pediatric OSA [13] [7] There are various other conditions that may lead to the cause of pediatric OSA which include but are not limited to genetic disorders and syndromes such as Downs syndrome, achondroplasia, Pierre Robin sequence, Apert syndrome, Crouzon syndrome, Treacher-Collins syndrome, Turner syndrome, Apert syndrome, Hemifacial microsomia, cleft palate, and cerebral palsy. [14] Children with Autism spectrum disorder have demonstrated significantly more sleep-related disorders. [15] When the symptom of snoring is unrelated to respiratory disorders, such as apnea, hypopnea, hypoxia, or hypercapnia, it is characterized as primary snoring. [16] It is very atypical that primary snoring leads to pediatric OSA and if the child's snoring is unrelated to respiratory disorders like hypoxia or apnea then it does indicate the need for any further intervention. [17]
2. Clinical Features of Pediatric OSA
- It is important to have a detailed history with the parents or the caregivers thoroughly to retrieve the most accurate clinical representation of the patients bearing in mind that the children themselves can only acknowledge and describe the symptoms when they are older. [18] The clinical features of OSA vary among different pediatric age groups and can be divided into four different categories: infants, toddlers, preschool-age children, and school-age children. [18] According to Guilleminault, the top two symptoms in children suffering from OSA are snoring and labored breathing. Besides the most common symptoms, children affected with OSA present with hypoxemia, hypoventilation, disruption pattern in sleep, and poor gas exchange. [19] Additionally, fatigue and somnolence during the day may also be noted, inclusive of chronic snoring. [20] Upon examination parents have mentioned night sweats, persistent snoring, paradoxical breathing, mouth breathing, disturbed sleep, drooling while sleeping, sleep terrors, sleepwalking, and bedwetting in children affected with OSA. [16] [18] [21] OSA-affected patients might present a variety of changes in their behavior based on their age group for example, toddlers and preschoolers characteristically exhibit hyperactivity, while school-age children usually exhibit aggression, difficulty learning, and below-average academic performance. [22] [23] It has been reported that children with OSA have a larger neck circumference than those without the condition. [8] Those with severe OSA in childhood may have developmental delays and also present with a failure to thrive. [2] Children who suffer from OSA frequently gasp for air, experience spells of trouble breathing, and breathe laboriously as they sleep, along with experiencing morning headaches, bedwetting, and hyperextended neck position. [21] [22].
3. Orofacial and Dental Changes in OSA
- To ensure a timely diagnosis, a pediatric dentist should be able to identify orofacial and dental changes associated with OSA. OSA patients with distinct craniofacial features involving a dolichofacial growth pattern (see figure 2), high and narrow arched palate, steep mandibular plane angle, and mandibular retrognathia have been noticed in various studies using cephalometric analysis and study models of dental casts of patients with OSA [24] As previously discussed, adenotonsillar hypertrophy is the leading cause of OSA and is often associated with Mallampati score classes III and IV. [25] The Mallampati classification, which is based on the examination of the upper airway, is a precise predictor of OSA and is observed by instructing the patient to open the mouth as wide as possible while protruding the tongue as far as possible. [26]. As described by Thorpy there are four classifications of Mallampati (see Figure 1 [27])• Class I: the soft palate and entire uvula are visible.• Class II: the soft palate, hard palate, and upper portion of the uvula are visible.• Class III: the soft palate, hard palate, and base of the uvula are visible.• Class IV: only the hard palate is visible.
 | Figure 1. Mallampati Classification [Source citation (27)] |
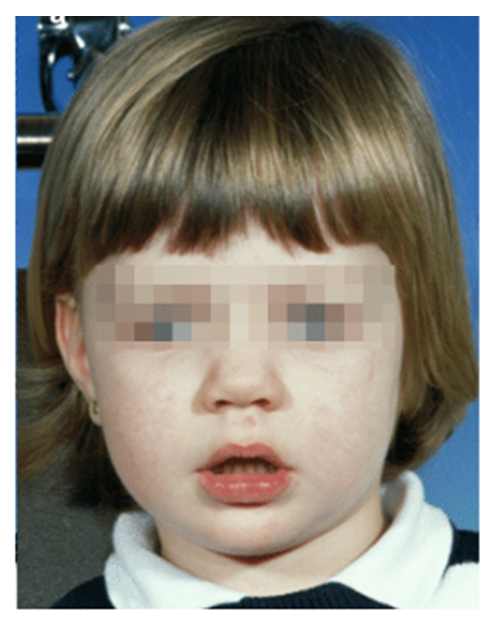 | Figure 2. Dolichofacial growth pattern (Source Citation [57]) |
4. Diagnosis
- Polysomnography (PSG), or sleep study, remains the gold standard for the diagnosis of OSA because it has been proven to calculate the ventilatory and sleep irregularities associated with sleep-disordered breathing. [36] Other diagnostic imaging studies may aid in evaluating underlying anatomical anomalies that can lead to OSA but are only valuable in combination with a PSG to make a conclusive diagnosis. [37] Other tools that can be used in the evaluation of OSA are overnight oximetry to provide additional information however it does not replace a PSG for a definitive diagnosis. [38]. A medical provider may proceed with a detailed investigation using a chest x-ray, echocardiogram, EKG, and biochemical markers to assess underlying anatomic abnormalities along with a PSG before the surgical management of OSA. [39] Kallikrein1, uromodulin, urocortin-3, and orosomucoid-1 emerge to be potential biomarkers for the assessment of OSA in children. [40]A dental provider must enquire the parents or the caregivers explicitly about signs and symptoms such as snoring and repeated changes in sleep posture as it is not often that the parents or caregivers may disclose information on the obvious signs and symptoms themselves. [41] If left untreated, OSA can result in problems, which may include neurocognitive deterioration, behavioral issues, failure to thrive, or cor pulmonale, predominantly in severe OSA cases. [42] Dentists should examine the clinical signs and symptoms and investigate about the characteristics of OSA before requesting a PSG. Depending on the results of this evaluation, a PSG recommendation or referral to a sleep specialist should come next. Children with OSA should have their oropharyngeal structure suitably assessed. [31] Adenotonsillar hypertrophy, which is the primary cause and predictor of OSA, must be examined during an oral cavity examination, followed by a Mallampati score that is significant enough to serve as an impartial predictor of OSA. [43] The tonsillar size is directly proportional to the severity of OSA, which means that the more the tonsillar size is, the more severe the OSA [39]. An increased Mallampati score also increases the risk of developing OSA and a surge of one point in the score will escalate the probability of OSA by sixfold. [43] A dental provider can refer for a PSG when a child presents with a high risk for OSA, including a history of chronic nocturnal snoring, adenotonsillar hypertrophy, and/or a high Mallampati score to confirm the diagnosis. It is noteworthy to mention that although hypercarbia is absent in the adult OSA population, it may be present in children when diagnosed with OSA and this is less prevalent in adults but is introduced by a prolonged hypoventilation state among children. [44] The apnea-hypopnea index (AHI) measured during PSG is 5 or more in adults, however, the equivalent index is 1 or more in the pediatric population. [19] Additionally, the degree of severity of OSA in children can be diagnosed by the AHI index and if the AHI is between 1.5 and 5, the OSA is measured as mild, while an AHI of more than 5 but less than 10 is determined to be moderate OSA, and an AHI more than 10 is considered severe OSA. [22] [19]
5. Management
- The management of OSA can be broadly classified into surgical and non-surgical treatment and the decision as to which treatment option is followed depends on the severity and underlying cause of OSA, and the patient’s ability to comply with the recommended treatment. [10]
5.1. Surgical Treatment
- The surgical treatments, including a variety of treatment modalities, such as adenotonsillectomy, adenoidectomy, and tonsillectomy, are presented to the patient to tackle the particular underlying cause and these treatment options may also incorporate the use of continuous positive airway pressure (CPAP) or intranasal corticosteroids to treat residual OSA. [6] A meta-analysis has also suggested maxillomandibular advancement in adults for the treatment of OSA however this invasive procedure has not been adequately studied in the pediatric population. [45] Patients with severe retrognathia associated with the Pierre Robin sequence or Treacher Collins syndrome are more likely to undergo this invasive surgical treatment. [45] Other surgical treatments that are more routinely implemented in adult patients with OSA, comprise uvulopalatopharyngoplasty, ablation, correction of previous posterior pharyngeal flap surgery, distraction osteogenesis, or tracheostomy however, there is a hesitancy to use these surgical procedures in children, with the exclusion of those with craniofacial abnormalities. [46]
5.2. Non-Surgical Treatment
- Oral appliance therapy (OAT) is supported by updated guidelines from the American Academy of Sleep Medicine (AASM), which state that it should be used "as a first-line therapy for mild and moderate OSA." [47] The recognition, diagnosis, and management of OSA with OAT are when both dental and medical providers should work together in the best interest of the patient. [19] The underlying issues of OSA in children have now been made clearer recently due to advancements in imaging techniques, such as cone beam computed tomography (CBCT), as well as the assimilation of dental and medical sciences. This insight has been valuable in establishing potential dental therapies for OSA, whose principal cause is dentofacial abnormalities. [6] After an adenotonsillectomy, only 25% of patients report relief from the symptoms of OSA and therefore dental treatments can be used alone, or in conjunction with other treatment alternatives like CPAP, to treat any residual OSA after an adenotonsillectomy [48] These dental treatment alternatives predominantly include growth-modifying appliances of the dentofacial region such as rapid maxillary expansion (RME) and mandibular growth activators, mandibular advancement appliances, and various other tongue positioning appliances. Myofunctional therapy is a treatment modality that targets to reinforce the orofacial structures by instructing patients on the preferred way to reposition their muscles to alleviate the symptoms of OSA. [32] Thus, it would appear that oral myofunctional therapy should be considered a routine part of an all-inclusive approach in the treatment of pediatric OSA to facilitate the proper orofacial and oropharyngeal development of a child. [32] A meta-analysis by Camacho et al. determined that myofunctional therapy reduces the AHI by almost 50% in adults and 62% in children. [49]Rapid Maxillary Expansion (RME)As previously discussed, children with OSA frequently have midfacial hypoplasia, a high-arched, narrow palate with crowding in the maxillary teeth. A rapid palatal expander has been shown to increase the transverse width of the nasal airway as well as has resulted in an expansion of the oropharyngeal air space, a decrease in nasal obstruction, and a raised posture of the tongue. [20] Besides the correction of dental occlusion and skeletal discrepancy, RME shows the potential to alleviate the symptoms of OSA [22] Another meta-analysis reports that there is a considerable decrease in the AHI after RME treatment in pediatric patients with OSA and suggested RME as a treatment alternative for OSA in children. [50]Mandibular AdvancementAs described earlier, mandibular retrognathia and Class II malocclusion have been noted in children with OSA. Multiple studies have established favorable results in treating these conditions, thereby increasing the overall oropharyngeal space. [51] [52] After treating class II patients, the authors of this study discovered a significant increase in pharyngeal airway space. [51] In a different study, it was indicated that treating a class II patient who has a retrognathic mandible can widen their airways and enhance their nighttime breathing. [53] Functional appliances, surgical correction, and mandibular repositioning are some of the approaches used to advance the mandible, and all these lead to an increase in the oropharyngeal space, alleviation of signs and symptoms of apnea, as well as a reduction in the AHI. [6] To treat patients diagnosed with OSA or avoid future impending risks, a guided treatment approach using a myofunctional appliance is preferable in children. [19]According to a systematic review [42], it has been concluded that Surgery is the best treatment option for AHI as the outcome measure and RME may help get the lowest arterial oxygen saturation. However, neither of the available treatments, but even so, has been demonstrated to completely relieve OSA by itself [42]. Conclusively, it is vital to highlight that the American Sleep Disorders Association states, “the presence or absence of OSA must be determined by a qualified physician before initiating treatment with oral appliances to identify those patients at risk due to complications of sleep apnea, and to provide a baseline to establish the effectiveness of subsequent treatment.” [19]
6. Discussion
- Healthcare professionals can now better understand the mechanism of OSA following recent advancements in sleep medicine and the increasing awareness of the many etiological factors for OSA. These advancements have also sparked the development of numerous unique treatment alternatives. Dental providers now recognize how minor deviations from anatomical structures, such as midfacial hypoplasia, cleft lip and palate, and a short lingual frenulum, could be potential risk factors for OSA. Advances in imaging techniques have emphasized the importance of orofacial variations in the development of OSA. [54] The role of a dental provider has been considerably impacted, and children seeking dental care must be examined for more than just teeth and they ought also to be evaluated for other potential future medical problems. The report by the National Academy of Sciences recommends a multi-disciplinary approach that includes dental providers along with medical practitioners owing to the excessive demand for the treatment of children with sleep-related breathing disorders and the significant scarcity of healthcare providers who can identify, diagnose and treat these children. [55] The American Academy of Pediatric Dentistry (AAPD) recognizes that cardiovascular complications, stunted growth and development (including failure to thrive), learning difficulties, and behavioral issues are all linked to undiagnosed or untreated OSA. [56]. The AAPD in its policy also urges dental providers to routinely examine their patients for increased risk of OSA and to provide medical referrals when required to minimize such complications. Pediatric dentists are in a remarkable position to recognize patients and monitor the mandibular and maxillary growth in the developing pediatric population. According to Stauffer, “the only dental practitioners currently considered qualified to treat OSA and fit mandibular advancement devices are Diplomates of the American Board of Dental Sleep Medicine or others who have undertaken comprehensive training in sleep medicine and/or sleep-related breathing disorders.” [19] Therefore, only the dental providers who have obtained additional training in this capacity should participate in this interdisciplinary role to prevent any adverse consequences in the management of OSA.
7. Conclusions
- Obstructive sleep apnea is becoming a common condition that affects the overall health of children as well as their quality of life. Therefore, a dental provider must be aware of the signs and symptoms of OSA and obtain a thorough history, extraoral and intraoral examination, and questionnaires, and appreciate the role of comorbid illnesses. Depending on this recent literature review, we suggest that the minimum requirements for present-day dental care provided to children should include education on the epidemiology, etiopathogenesis, and treatment alternatives of children with OSA. By conducting routine history-taking, questionnaires, and clinical examinations, and making the appropriate referrals to the medical practitioners, dental providers may also get involved in identifying OSA. Also, a dentist may choose to pursue the appropriate continuing or higher education required for diagnosing OSA, fabricating, and delivering oral appliances for the treatment of OSA. It has to be reinstated that a multidisciplinary treatment approach often is the most beneficial for the patient.
8. Limitations
- The limitation of this paper is the bias of the individual cited literature. The degrees of evidence in the cited studies have not been offered for review. We have also not specified a rating of evidence for each source; therefore, the reader should consider the strength of evidence for each subject.
9. Disclosures
- The authors report no specific grants by funding organizations in the public, private, or not-for-profit sectors. None of the authors have any financial or personnel conflicts of interest.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML