-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2023; 13(6): 825-830
doi:10.5923/j.ajmms.20231306.11
Received: May 25, 2023; Accepted: Jun. 22, 2023; Published: Jun. 26, 2023

Serum Level of Neurotropic Autoantibodies in COVID-19 Associated Ischemic Stroke Patients
Rasulova M. A., Rasulova Kh. A.
Tashkent Pediatric Medical Institute, Uzbekistan
Correspondence to: Rasulova M. A., Tashkent Pediatric Medical Institute, Uzbekistan.
| Email: |  |
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Since 2019, the whole world has been experiencing a pandemic of coronavirus infection. However, unlike previous outbreaks, COVID-19 has a high virulence and pathogenicity, and is also spreading around the world at lightning speed. In this connection, a pandemic was declared by the World Health Organization (WHO) on March 11, 2020. At the moment, there is an increase in the number of patients with COVID 19 around the world who develop coagulation disorders and a high prevalence of thromboembolic complications. Neurological syndromes caused by the production of antibodies to nervous tissue during COVID-19 coronavirus infection are a new area of modern clinical neurology, which is of great interest from theoretical and practical positions.
Keywords: COVID-19, Ischemic stroke, Immune system
Cite this paper: Rasulova M. A., Rasulova Kh. A., Serum Level of Neurotropic Autoantibodies in COVID-19 Associated Ischemic Stroke Patients, American Journal of Medicine and Medical Sciences, Vol. 13 No. 6, 2023, pp. 825-830. doi: 10.5923/j.ajmms.20231306.11.
1. Introduction
- Neurological syndromes caused by the production of antibodies to nervous tissue during COVID -19 coronavirus infection are a new area of modern clinical neurology, which is of great interest from theoretical and practical positions. Significantly affecting the immune system, COVID -19 causes autoimmune and metabolic changes that occur in the human body when an infection enters both in the acute and late (post-COVID) period [9,23,24,26].One of the well-documented additional clinical manifestations of COVID-19 is acute cerebrovascular accident (ACV) [7,21]. Ischemic stroke (IS), secondary to severe COVID-19, is common and often fatal. Exploring the mechanisms by which SARS-CoV-2 induces AI has become a popular research topic. Even the first L. Mao et al. (2020) demonstrated that patients with severe COVID-19 were more likely to develop complications of IS, which were associated with higher mortality rates [23].It is known that various functional states of the body are accompanied by shifts in the content of natural autoantibodies (e-AT), associated with changes in metabolic processes of key endogenous targets for the development of the disease or providing a physiological norm. Similar studies on the analysis of the content of n-AT were carried out in various neurological and psychiatric diseases: ischemic [2,11,15,16] and hemorrhagic stroke [29], epilepsy [14], schizophrenia and psychosis [10], dementia, Alzheimer's disease [5,6], chronic alcohol intoxication [1], neurodegenerative diseases [17,18], encephalitis [19], and others.Significant variability of pathogenetic events that occur with COVID -19 determines the multiorganism of the lesion, in the development of which the immunopathogenic properties of SARS-CoV-2 can also play a certain role. Autoantibodies associated with a number of autoimmune diseases have been found in patients with COVID-19 [20,22,25,27]. Researchers have identified the presence of antinuclear antibodies (ANA), anticytoplasmic neutrophil antibodies (ANCA) and anti-antiphospholipid (APL) antibodies in patients with COVID-19. The results showed that 45% of patients were positive for at least one autoantibody, and patients with positive autoantibodies tended to have a worse prognosis and a significantly higher respiratory rate on admission. The positive rate for ANA was 33%, the positive rate for anticardiolipin antibodies (IgG and/or IgM) was 24%, and three patients were positive for antibodies against β2-glycoprotein-I (IgG and/or IgM) (9%). However, ANCA was negative in all patients [13,20,28].Analysis of the content of n-ATs, which preserve all changes in the EB system in patients with COVID-19 associated IS, will allow solving the problem of diagnosing and treating neurological syndromes in COVID-19.The purpose of the study: was to conduct a comparative analysis of the content of natural neurotropic autoantibodies in the blood serum of patients with COVID-19 associated IS in the course of the disease.
2. Materials and Methods
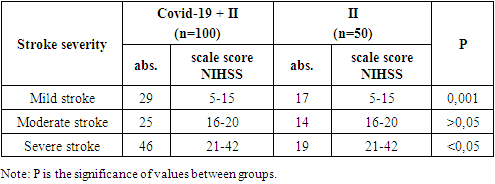
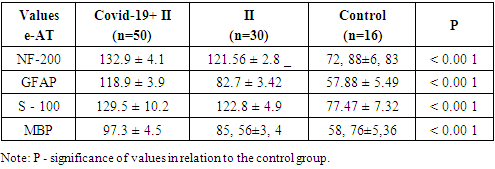
- This study was carried out at the Department of Internal Medicine, Nephrology and Hemodialysis of TashPMI in the period 2020-2022. The basis of the clinical study included the results of monitoring 150 patients in acute and early recovery periods of IS, of which 100 patients were with IS due to COVID-19 pneumonia (main group or group 1) and 50 patients with acute IS without symptoms and a positive test for COVID -19 (comparison group or group 2). Clinical studies were carried out in the departments of resuscitation and intensive care, neurology of the Specialized Multidisciplinary Infectious Diseases Hospitals Zangiota-1 and Zangiota-2, as well as the City Clinical Hospital No. 5 in Tashkent.The age of patients in group 1 (57 males) and (43 females) ranged from 41 to 89 years (mean age) 68.3±9.8 years in group 2 (27 males) and (23 females) - from 42 to 83 years (mean age 64.2±10.2 years).The control group consisted of 16 people without stroke and COVID-19, aged 50 to 68 years (mean age 61.2±5.7 years) with stage 1 dyscirculatory encephalopathy, whose data were used to compare immunological parameters.The criteria for inclusion in the study of patients of the main group were the first acute IS, the transferred coronavirus infection COVID -19 for up to 1 month; comparison groups: new acute IS, absence of clinical symptoms and a positive test for COVID -19, absence of COVID -19 infection before the onset of IS (included patients with IS before March 2020).Criteria for exclusion of patients from the study: hemorrhagic stroke, late recovery period and consequences of ischemic stroke, recurrent stroke, presence of neurocognitive disorders before COVID-19 and stroke, neurodegenerative and extrapyramidal diseases, severe TBI, epilepsy, mental and oncological diseases, severe somatic diseases in the stage of decompensation.All patients underwent a detailed clinical and neurological examination according to the classical method. The assessment of consciousness and the severity of its impairment was carried out using the Glasgow coma scale. The severity of neurological deficit and the severity of IS were assessed using the Stroke Severity Scale of the US National Institutes of Health (National Institutes of Health Stroke Scale - NIHSS). Assessment of the condition of patients was carried out once at the time of the initial examination in clinics on days 1-5, 14, 28 of the disease.The formulation of the diagnosis of COVID -19 associated IS was carried out on the basis of the results of an epidemiological history, clinical and neurological examination and data from laboratory and instrumental studies in accordance with the criteria of the ICD-10 (U07.1 - U07.2), the National Guidelines for Neurology and generally accepted documents (European Stroke Organization (ESO) Executive committee; ESO Writing Committee, Guidelines for management of ischemic stroke and transient ischemic attack, 2008), Temporary Guidelines of the Ministry of Health of Russia, "Prevention, diagnosis and treatment of a new coronavirus infection COVID-19" [3,4]. To identify COVID-19 pneumonia, its complications, differential diagnosis with other lung diseases, as well as to determine the severity and dynamics of changes, to evaluate the effectiveness of the therapy, chest MSCT was performed. According to the examination standards, to clarify the nature of the pathological process and exclude hemorrhagic stroke, all patients underwent brain CT upon admission to the clinic.The basic laboratory examination of inpatients included standard general clinical and biochemical blood tests, a detailed coagulogram, and a general urinalysis. According to the COVID -19 Diagnostic Protocols, the levels of C-reactive protein (CRP), procalcitonin, D-dimer, ferritin, fibrinogen, and triglycerides were also examined. The detection of class M and G immunoglobulins (IgM and IgG) using immunochemical methods was of primary importance for the etiological laboratory diagnosis of COVID-19. The main type of biomaterial for laboratory testing by PCR for SARS-CoV-2 RNA was the material obtained by taking a swab from the nasopharynx (from two nasal passages) and oropharynx. Swabs from the mucous membrane of the nasopharynx and oropharynx were collected in one tube for a higher concentration of the virus.In blood serum samples of all observed patients with IS, as well as in blood samples of the control group (n = 16), a quantitative determination of serum immunoreactivity of neurotropic autoantibodies of the IgG class (natural neurotropic autoantibodies - e-AT1 and their functional counterweights - anti-idiotypic antibodies - AiAT2) was carried out, directed to nervous tissue proteins NF-200, GFAP, S-100, MBP.Determination of the content of neurotropic autoantibodies (NAAT) was carried out using standard procedures for enzyme-linked immunosorbent assay ELI-N-Test and test kits of the same name produced by the Immunculus Medical Center (Russia) according to the method of A.B. Poletaev [11,12]. The level of serum content of n-AT to each of the neuroantigens was expressed in arbitrary units (arb. units): percentage deviations from the standard serum IR. AAT immunoreactivity values from 80 to 140 c.u., AT1/AIAT2 immunoreactivity index from 0.8 to 1.2 were taken as the norm [14]. "ELI-tests" can be detected when signs of blood are detected, detection is still on that detection of development. This test allows patients to assess the state of the central and peripheral nervous system by antibody markers. The study is especially significant when a person is at risk (hereditary predisposition) to severe pathologies of the nervous system, such diseases as stroke, amyotrophic lateral (lateral) sclerosis.The results obtained were recorded in individual patient registration cards and then entered into the electronic database of the Microsoft Excel 2010 program. The generally accepted methods of variation statistics were used. The results are presented as M (mean) ± m (error) and µ (mean) ± (standard deviation). After confirming the normality of the data distribution, the analysis of quantitative indicators was carried out using Student's t -test. Differences were considered statistically significant at a significance level of at least 95% (p<0.05). The degree of connection between the obtained indicators was determined by the regression equation, taking into account the strength of the connection and its direction by calculating the correlation coefficient (r) according to Pearson.
3. Results and Discussion
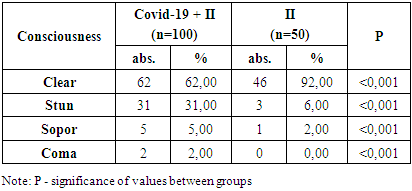
- The consciousness of patients with COVID-19 associated IS was clear in 62 (%) patients (mean Glasgow score), deafened in 31 (%) patients (mean Glasgow score), soporous in 5 (%) patients (mean Glasgow score). 2 (%) patients were in a coma (mean score on the Glasgow scale) (Table 1).
|
|
|
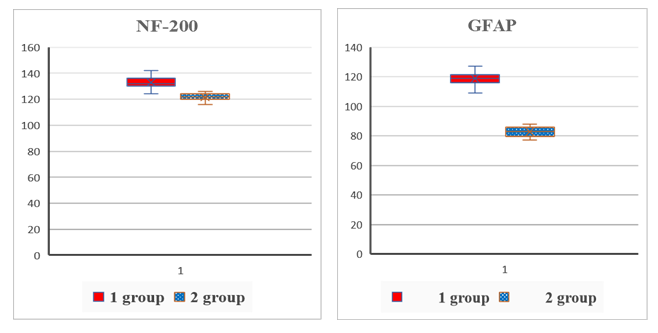 | Figure 1. Comparative content of autoantibodies to NF-200 and GFAP in the blood serum of the examined patients (conventional units) |
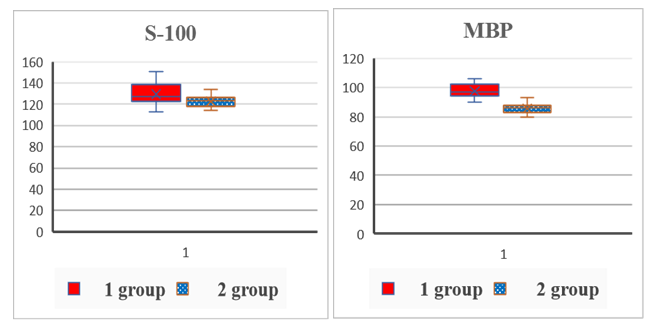 | Figure 2. Comparative content of autoantibodies to S-100 and MBP proteins in the blood serum of the examined patients (conventional units) |
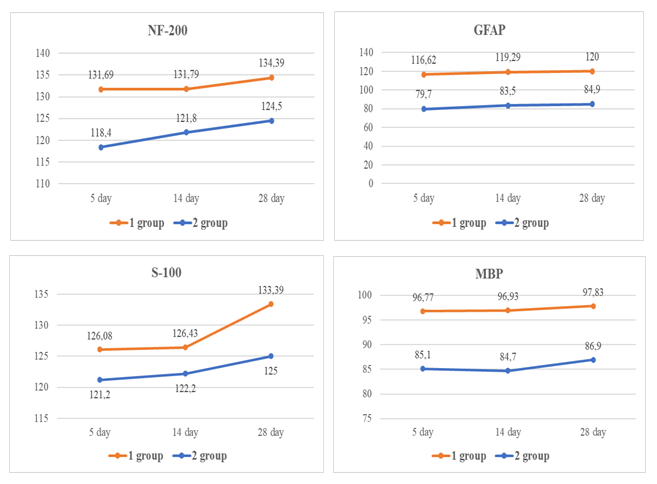 | Figure 3. Analysis of blood serum samples in patients (on 5-th,14-th and 28-th day) |
4. Conclusions
- 1. The study of the level of n-AT in patients with COVID -19 associated IS in the dynamics of the disease showed the greatest increase in the level of n-AT on day 28 to protein S-100, NF-200 and MBP, which may well explain the variety of symptoms of COVID-19 and long-term consequences coronavirus infection (post-covid syndromes) in patients with acute cerebrovascular accidents.2. In patients with COVID-19 associated ischemic stroke, there were differences in the content of natural neurotropic autoantibodies in blood serum compared to patients with IS without COVID-19. In patients with IS against the background of COVID-19 pneumonia, a more enhanced production of serum autoantibodies to neuroproteins was revealed, which accompanied a worse course of IS and can be considered as a predictor of an unfavorable outcome of the disease.3. The evidence obtained for the participation of natural neurotropic antibodies expands the current understanding of the dysregulatory mechanisms of neuroimmune interactions in COVID-19 and may further form the basis for the development of additional immunotherapy for this disease.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML