-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Medicine and Medical Sciences
p-ISSN: 2165-901X e-ISSN: 2165-9036
2023; 13(6): 810-813
doi:10.5923/j.ajmms.20231306.08
Received: May 28, 2023; Accepted: Jun. 17, 2023; Published: Jun. 21, 2023

Evaluation of the Effectiveness of Bcf-Based Eradication Therapy in Patients with Helicobakter Pylori Associated Chronic Gastritis
Karimov M. M.1, Karimova D. K.1, Sobirova G. N.2, Abdullayeva U. K.3
1State Institution “Republican Specialized Scientific and Practical Medical Center for Therapy and Medical Rehabilitation”, Tashkent, Uzbekistan
2Tashkent Medical Academy, Tashkent, Uzbekistan
3Bukhara State Medical Institute, Bukhara, Uzbekistan
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The article presents data on the results of a comparative study of the clinical and anti-helicobacter efficacy of two schemes of eradication therapy in 60 patients with HP-associated chronic gastritis. The main group of patients received eradication therapy consisting of vonoprazane, amoxicillin, clarithromycin and bismuth tricalium decitrate for 10 days. In the second group of patients with chronic gastritis, esomeprazole was used as an antisecretory drug against the background of similar therapy. The results of the study showed that in the first group of patients, the dynamics of acid suppression under the influence of vonoprase was significantly higher compared to esomeprazole. Also, in the main group of patients, the rate of HP infection eradication was 93%, whereas in the group of patients taking esomeprazole, this indicator was 80%.
Keywords: Vonoprazan, K-KBK, Helicobacter pylori, Chronic gastritis, Eradication
Cite this paper: Karimov M. M., Karimova D. K., Sobirova G. N., Abdullayeva U. K., Evaluation of the Effectiveness of Bcf-Based Eradication Therapy in Patients with Helicobakter Pylori Associated Chronic Gastritis, American Journal of Medicine and Medical Sciences, Vol. 13 No. 6, 2023, pp. 810-813. doi: 10.5923/j.ajmms.20231306.08.
1. Introduction
- After the discovery by R. Warren and B. Marshall of the role and significance of Helicobacter pylori (HP), which plays a leading role in the pathogenesis of chronic gastritis (PCG), duodenitis, gastric and duodenal ulcer, gastric adenocarcinoma, as well as gastric malthymphoma, the Masstricht Consensus and subsequently the Kyoto Protocol on the Diagnosis and Treatment of the above diseases were formed. The evolution of the Mastricht Agreements since 1996 and its revisions in 2000 ("Maastricht II"), 2005 ("Maastricht III") and 2010 ("Maastricht IV") 2015 [1,2,3]. ("Maastricht V") defined the basic principles of diagnosis and treatment of HP-associated diseases for two decades and had a significant impact on clinical gastroenterology. They were based on the use of proton pump inhibitors (PPIs), amoxicillin, clarithromycin and imidazoles. Although this treatment regimen showed good results in the eradication of HP, subsequently the effectiveness of this type of therapy began to decrease due to the formation of resistance resistant to the antibacterial drugs used. This problem was partially solved by the use of new-generation PPIs in doubled doses, the addition of bismuth preparations to the treatment regimen, the lengthening of the duration of treatment from 7 to 10-14 days, the use of additional second- and third-line treatment regimens, the possible use of pre/probiotics [4]. Despite a partial solution to the problem of antibiotic resistance, it has not been possible to completely solve this problem until today. The Maastricht VI protocols published in 2022 for the first time formulated a conclusion on the effectiveness of a new class of antisecretory agents – potassium channel blockers (P-CAB) [5]. It is indicated that potassium channel blockers in combination therapy are superior to traditional PPIs or are not inferior to them in triple therapy of the first and second lines and have an advantage in patients with antibiotic-resistant infection. (Consistency 100%, evidence B2). Vonoprazane is the first registered potassium-competitive acid blocker (K-KBK). Like PPIs, K-KBCS block the final stage of acid formation of the parietal cell — H+, K+-ATPase. However, unlike IPN, which realize their acid-suppressive effect due to covalent binding to cysteine groups of H+, K+-ATPase, K-KBK competitively interact with the ionic K+-binding domain of H+, K+-ATPase. All K-KBCS are acid-stable, lipophilic, weak bases with high ionization constants (pKa), varying depending on the drug from 5.6 to 9.06. This ensures a very high accumulation of the active substance in the secretory tubules of the parietal cell. All the described properties allow K-KBK to have a longer and faster antisecretory effect [6]. Since 2020, Vonoprazane has been included in the ATC, in the group "A02BC PPIs", and the code A02BC08 has been assigned to it, and two combinations of vonoprazane with antimicrobial agents have been assigned codes and they have also been included in the ATC, in the group "A0BD Combinations of drugs for eradication of Helicobacter pylori" since 2020. During studies conducted in Japan, it was found that within the framework of triple eradication therapy of the first line, vonoprazane provides a better rate of HP eradication (about 98%) than lansoprazole (about 76%) in patients with gastric and duodenal ulcer [7]. Similarly, vonoprazane demonstrated a better frequency of eradication in combination with the same antibiotics as part of second-line therapy p[8]. This is explained by the fact that vonoprazane can maintain the pH of the stomach in the region of neutral values longer than PPIs, which cannot provide such acidity during the day, regardless of the dose and frequency of administration [9,10].In order to assess the antisecretory and antihelicobacteric efficacy, a comparative study was conducted with two parallel groups of patients with a verified diagnosis of chronic HP-associated gastritis B based on antisecretory drugs of the PPIs and K-KBK groups.
2. Material and Methods
- 60 patients with a verified diagnosis of chronic HP-associated gastritis type B (32 men and 28 women, average age 34.5+ 4.3 years) were examined. The diagnosis was verified on the basis of EFGDS with targeted biopsy. HP infection was diagnosed on biopsies with a rapid urease test (AMA RUT Pro). The degree of atrophy of the gastric mucosa was carried out according to a test with pepsinogens I and II (chronic non-atrophic gastritis was detected in 48% of patients and chronic atrophic gastritis in 52% of patients). The acid-forming function of the stomach was studied by transedoscopic, topographic pH-metry (AGM-03). The patients were divided into two groups, comparable in gender, age, and features of the course of chronic gastritis. The main group of patients (30 people) took the following scheme of eradication therapy: vonoprazan (voniza Hilton Pharma (Pvt.) Ltd) 20 mg 2 times a day, amoxicillin 1.0 grams 2 times a day, clarithromycin 500 mg 2 times a day and bismuth potassium decitrate 120 mg 4 times per day for 10 days. The control group also received eradication therapy based on esomeprazole 40 mg 2 times a day, amoxicillin 1.0 grams 2 times a day, clarithromycin 500 mg. Per day and bismuth tricalium decitrate 120 mg 4 times a day. HP eradication was monitored 4 weeks after the end of the course of therapy with a non-invasive, respiratory C14 urease test (HUBT-20P, Headway). The obtained results of the study were processed by the method of variational statistics using a package of statistical programs.
3. Results and Discussion
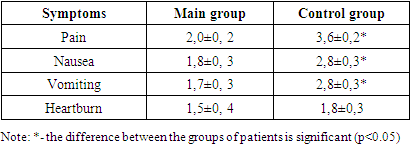
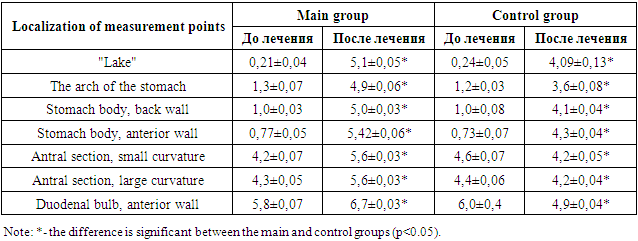
- The clinic of pain symptoms in patients consisted of "hungry", "late" and "night" pains. The totality of these symptoms was observed in almost all examined patients of both the main and control groups. Dyspeptic symptoms in patients consisted of symptoms such as nausea, vomiting and decreased appetite. The topographic, tranendoscopic pH-metry of the patients showed the phenomena of hyperacidity of gastric juice (Table 1). This was expressed in a decrease in the pH index in the zone of active acid formation (the front and back walls of the stomach body and the "lake" below 2.0). This indicated that in chronic gastritis and peptic ulcer disease, there are sub- and decompensated violations of the process of neutralization of hydrochloric acid in gastric juice. At the same time, the alkalizing function of the antrum was considered preserved at pH > 5 in the middle third of the antrum.
|
|
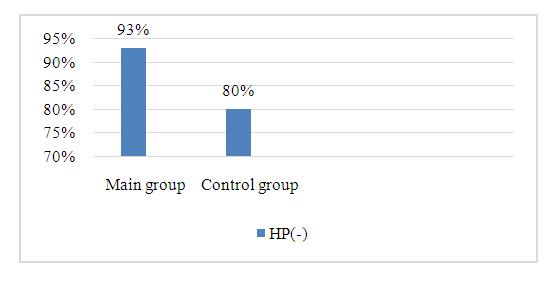 | Figure 1. Indicators of the effectiveness of eradication therapy based on the results of the C-14 breath test |
4. Conclusions
- 1. When using vonoprazane in the complex of eradication therapy of patients with HP-associated chronic gastritis, a higher rate of relief of abdominal pain and dyspeptic symptoms was observed.2. The drug vonoprazane showed higher antisecretory activity in patients with HP-associated chronic gastritis compared with esomeprazole, which was expressed by significantly higher effects in terms of increasing pH in the area of active acid production, and in normalization of acid neutralization processes in the stomach.3. Eradication therapy of patients with HP-associated chronic gastritis using vonoprazane as an antisecretory drug showed a close to 95% eradication rate, which according to the VI Maastricht Protocols is assessed as an “excellent” result.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML